Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis
- PMID: 17591857
- PMCID: PMC2118628
- DOI: 10.1084/jem.20070618
Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis
Abstract
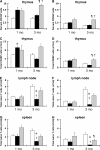
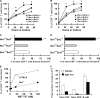
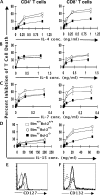
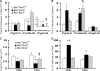
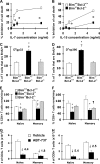
We examined the role of the antiapoptotic molecule Bcl-2 in combating the proapoptotic molecule Bim in control of naive and memory T cell homeostasis using Bcl-2(-/-) mice that were additionally deficient in one or both alleles of Bim. Naive T cells were significantly decreased in Bim(+/-)Bcl-2(-/-) mice, but were largely restored in Bim(-/-)Bcl-2(-/-) mice. Similarly, a synthetic Bcl-2 inhibitor killed wild-type, but not Bim(-/-), T cells. Further, T cells from Bim(+/-)Bcl-2(-/-) mice died rapidly ex vivo and were refractory to cytokine-driven survival in vitro. In vivo, naive CD8(+) T cells required Bcl-2 to combat Bim to maintain peripheral survival, whereas naive CD4(+) T cells did not. In contrast, Bim(+/-)Bcl-2(-/-) mice generated relatively normal numbers of memory T cells after lymphocytic choriomeningitis virus infection. Accumulation of memory T cells in Bim(+/-)Bcl-2(-/-) mice was likely caused by their increased proliferative renewal because of the lymphopenic environment of the mice. Collectively, these data demonstrate a critical role for a balance between Bim and Bcl-2 in controlling homeostasis of naive and memory T cells.
Figures

References
-
- Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

