Fascicular specialization in human and monkey rectus muscles: evidence for anatomic independence of global and orbital layers
- PMID: 17591878
- PMCID: PMC1978188
- DOI: 10.1167/iovs.06-0692
Fascicular specialization in human and monkey rectus muscles: evidence for anatomic independence of global and orbital layers
Abstract
Purpose: Connective tissue pulleys inflect the extraocular muscles (EOMs) and receive insertions from some fibers. The authors investigated insertions and anatomic relationships of fiber fascicles within rectus EOMs to clarify the relationship to their pulleys.
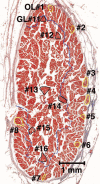
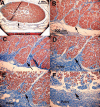
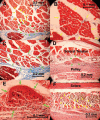
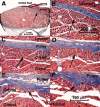
Methods: Two human and two monkey orbits were removed intact, serially sectioned in the coronal plane, histologically stained, and digitally photographed. The authors traced representative fascicles in the human medial rectus (MR) and inferior rectus and monkey lateral rectus and superior rectus muscles. In the human MR, the authors computed average collagen fractions in the orbital layer (OL) and the global layer (GL).
Results: In human and monkey, OL fascicles remained distinct from each other and from the GL throughout. Most OL fascicles were inserted into the pulley through short tendons. Most GL fascicles bypassed the pulley without insertion. Collagen content in the human MR OL increased from 29% +/- 5% (SD) in midorbit to 65% +/- 9% in the anterior orbit but slightly decreased from 26% +/- 6% to 23% +/- 1% in the GL. Tracing of every fiber in a human MR OL fascicle demonstrated terminations on pulley tendons without myomyous junctions.
Conclusions: Fibers in the primate rectus OL lack myomyous or GL junctions, but nearly all insert on the pulley through a broad distribution of short tendons and dense intercalated collagen. Fibers in the GL generally do not insert on pulley tissues and are associated with less collagen. These features support the distinct role of the OL in anteroposterior positioning of connective tissues proposed in the active pulley hypothesis and substantial mechanical independence of the OL and GL.
Figures




Similar articles
-
Quantitative analysis of rectus extraocular muscle layers in monkey and humans.Invest Ophthalmol Vis Sci. 2001 Jan;42(1):10-6. Invest Ophthalmol Vis Sci. 2001. PMID: 11133842
-
Superior oblique muscle layers in monkeys and humans.Invest Ophthalmol Vis Sci. 2005 Aug;46(8):2790-9. doi: 10.1167/iovs.04-1147. Invest Ophthalmol Vis Sci. 2005. PMID: 16043852
-
Evidence for active control of rectus extraocular muscle pulleys.Invest Ophthalmol Vis Sci. 2000 May;41(6):1280-90. Invest Ophthalmol Vis Sci. 2000. PMID: 10798641
-
Double insertions of extraocular rectus muscles in humans and the pulley theory.J Anat. 2005 Mar;206(3):295-306. doi: 10.1111/j.1469-7580.2005.00383.x. J Anat. 2005. PMID: 15733302 Free PMC article. Review.
-
The orbital pulley system: a revolution in concepts of orbital anatomy.Ann N Y Acad Sci. 2002 Apr;956:17-32. doi: 10.1111/j.1749-6632.2002.tb02805.x. Ann N Y Acad Sci. 2002. PMID: 11960790 Review.
Cited by
-
Independent passive mechanical behavior of bovine extraocular muscle compartments.Invest Ophthalmol Vis Sci. 2012 Dec 19;53(13):8414-23. doi: 10.1167/iovs.12-10318. Invest Ophthalmol Vis Sci. 2012. PMID: 23188730 Free PMC article.
-
Magnetic resonance imaging demonstrates compartmental muscle mechanisms of human vertical fusional vergence.J Neurophysiol. 2015 Apr 1;113(7):2150-63. doi: 10.1152/jn.00871.2014. Epub 2015 Jan 14. J Neurophysiol. 2015. PMID: 25589593 Free PMC article.
-
Formation and Fixation of the Annulus of Zinn and Relation With Extraocular Muscles: A Plastinated Histologic Study and Its Clinical Significance.Invest Ophthalmol Vis Sci. 2022 Nov 1;63(12):16. doi: 10.1167/iovs.63.12.16. Invest Ophthalmol Vis Sci. 2022. PMID: 36355368 Free PMC article.
-
Author Response: Letter to the Editor of IOVS From Joseph L. Demer and Robert A. Clark Regarding Joel M. Miller, "EOM Pulleys and Sequelae: A Critical Review".Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):9. doi: 10.1167/iovs.61.6.9. Invest Ophthalmol Vis Sci. 2020. PMID: 32503049 Free PMC article. No abstract available.
-
Composition, Architecture, and Functional Implications of the Connective Tissue Network of the Extraocular Muscles.Invest Ophthalmol Vis Sci. 2018 Jan 1;59(1):322-329. doi: 10.1167/iovs.17-23003. Invest Ophthalmol Vis Sci. 2018. PMID: 29346490 Free PMC article.
References
-
- Demer JL, Miller JM, Poukens V, Vinters HV, Glasgow BJ. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. - PubMed
-
- Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240. - PubMed
-
- Sappey PC. Traite d'Anatomie Descriptive avec Figures Intercalees dans le Texte. Delahaye et Lecrosnier; Paris: 1888.
-
- Sappey PC. The motor muscles of the eyeball [in French] Strabismus. 2001;9:243–253. - PubMed
-
- Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci. 1997;38:1774–1785. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

