Radiogenic lymphangiogenesis in the skin
- PMID: 17591978
- PMCID: PMC1941592
- DOI: 10.2353/ajpath.2007.060589
Radiogenic lymphangiogenesis in the skin
Abstract
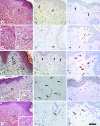
The time course of microvascular changes in the environment of irradiated tumors was studied in a standardized human protocol. Eighty skin biopsies from 40 patients with previously treated primary breast cancer were taken from irradiated skin and corresponding contralateral unirradiated control areas 2 to 8 weeks, 11 to 14 months, or 17+ months after radiotherapy (skin equivalent dose 30 to 40 Gy). Twenty-two biopsies of 11 melanoma patients who had undergone lymph node dissection were used for unirradiated control. We found an increase of total podoplanin(+) lymphatic microvessel density resulting mainly from a duplication of the density of smallest lymphatic vessels (diameter <10 microm) in the samples taken 1 year after radiation. Our findings implicate radiogenic lymphangiogenesis during the 1st year after therapy. The numbers of CD68(+) and vascular endothelial growth factor-C(+) cells were highly elevated in irradiated skin in the samples taken 2 to 8 weeks after radiotherapy. Thus, our results indicate that vascular endothelial growth factor-C expression by invading macrophages could be a pathogenetic route of induction of radiogenic lymphangiogenesis.
Figures

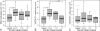
References
-
- Archambeau JO, Ines A, Fajardo LF. Correlation of the dermal microvasculature morphology with the epidermal and the endothelial population changes produced by single X ray fractions of 1649, 2231 and 2619 rad in swine. Int J Radiat Oncol Biol Phys. 1985;11:1639–1646. - PubMed
-
- Takahashi S, Sugimoto M, Kotoura Y, Sasai K, Oka M, Yamamuro T. Long-term changes in the haversian systems following high-dose irradiation: an ultrastructural and quantitative histomorphological study. J Bone Joint Surg Am. 1994;76:722–738. - PubMed
-
- Tsai JH, Makonnen S, Feldman M, Sehgal CM, Maity A, Lee WM. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther. 2005;4:1395–1400. - PubMed
-
- Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. - PubMed
-
- Reinhold HS. The influence of radiation on blood vessels and circulation. Chapter IV. Structural changes in blood vessels. Curr Top Radiat Res Q. 1974;10:58–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

