A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore
- PMID: 17591984
- PMCID: PMC2154364
- DOI: 10.1085/jgp.200709755
A Na+ channel mutation linked to hypokalemic periodic paralysis exposes a proton-selective gating pore
Abstract
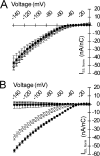
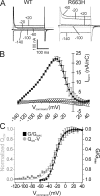
The heritable muscle disorder hypokalemic periodic paralysis (HypoPP) is characterized by attacks of flaccid weakness, brought on by sustained sarcolemmal depolarization. HypoPP is genetically linked to missense mutations at charged residues in the S4 voltage-sensing segments of either CaV1.1 (the skeletal muscle L-type Ca(2+) channel) or NaV1.4 (the skeletal muscle voltage-gated Na(+) channel). Although these mutations alter the gating of both channels, these functional defects have proven insufficient to explain the sarcolemmal depolarization in affected muscle. Recent insight into the topology of the S4 voltage-sensing domain has aroused interest in an alternative pathomechanism, wherein HypoPP mutations might generate an aberrant ionic leak conductance by unblocking the putative aqueous crevice ("gating-pore") in which the S4 segment resides. We tested the rat isoform of NaV1.4 harboring the HypoPP mutation R663H (human R669H ortholog) at the outermost arginine of S4 in domain II for a gating-pore conductance. We found that the mutation R663H permits transmembrane permeation of protons, but not larger cations, similar to the conductance displayed by histidine substitution at Shaker K(+) channel S4 sites. These results are consistent with the notion that the outermost charged residue in the DIIS4 segment is simultaneously accessible to the cytoplasmic and extracellular spaces when the voltage sensor is positioned inwardly. The predicted magnitude of this proton leak in mature skeletal muscle is small relative to the resting K(+) and Cl(-) conductances, and is thus not likely to fully account for the aberrant sarcolemmal depolarization underlying the paralytic attacks. Rather, it is possible that a sustained proton leak may contribute to instability of V(REST) indirectly, for instance, by interfering with intracellular pH homeostasis.
Figures



References
-
- Bulman, D.E., K.A. Scoggan, M.D. van Oene, M.W. Nicolle, A.F. Hahn, L.L. Tollar, and G.C. Ebers. 1999. A novel sodium channel mutation in a family with hypokalemic periodic paralysis. Neurology. 53:1932–1936. - PubMed
-
- Cannon, S.C. 2006. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu. Rev. Neurosci. 29:387–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

