Zebrafish model for human long QT syndrome
- PMID: 17592134
- PMCID: PMC2040896
- DOI: 10.1073/pnas.0702724104
Zebrafish model for human long QT syndrome
Abstract
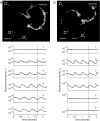
Long QT syndrome (LQTS) is a disorder of ventricular repolarization that predisposes affected individuals to lethal cardiac arrhythmias. To date, an appropriate animal model of inherited LQTS does not exist. The zebrafish is a powerful vertebrate model used to dissect molecular pathways of cardiovascular development and disease. Because fundamental electrical properties of the zebrafish heart are remarkably similar to those of the human heart, the zebrafish may be an appropriate model for studying human inherited arrhythmias. Here we describe the molecular, cellular, and electrophysiological basis of a zebrafish mutant characterized by ventricular asystole. Genetic mapping and direct sequencing identify the affected gene as kcnh2, which encodes the channel responsible for the rapidly activating delayed rectifier K(+) current (I(Kr)). We show that complete loss of functional I(Kr) in embryonic hearts leads to ventricular cell membrane depolarization, inability to generate action potentials (APs), and disrupted calcium release. A small hyperpolarizing current restores spontaneous APs, implying wild-type function of other ionic currents critical for AP generation. Heterozygous fish manifest overt cellular and electrocardiographic evidence for delayed ventricular repolarization. Our findings provide insight into the pathogenesis of homozygous kcnh2 mutations and expand the use of zebrafish mutants as a model system to study human arrhythmias.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

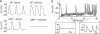
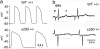
References
-
- Sehnert AJS, Stainier DYR. Trends Genet. 2002;18:419–424. - PubMed
-
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, et al. Circulation. 2000;102:1178–1185. - PubMed
-
- Hoorntje T, Alders M, van Tintelen P, van der Lip K, Sreeram N, van der Wal A, Mannens M, Wilde A. Circulation. 1999;100:1264–1267. - PubMed
-
- Schwartz PJ, Stramba-Badiale M. Prolonged Repolarization and Sudden Infant Death Syndrome. Philadelphia: Saunders; 2004.
-
- Babij P, Askew GR, Nieuwenhuijsen B, Su CM, Bridal TR, Jow B, Argentieri TM, Kulik J, DeGennaro LJ, Spinelli W, et al. Circ Res. 1998;83:668–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

