Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht's enzyme Y
- PMID: 17592142
- PMCID: PMC2040881
- DOI: 10.1073/pnas.0703714104
Chymotrypsin C (caldecrin) promotes degradation of human cationic trypsin: identity with Rinderknecht's enzyme Y
Abstract
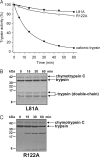
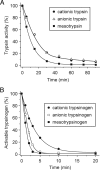
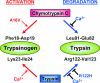
Digestive trypsins undergo proteolytic breakdown during their transit in the human alimentary tract, which has been assumed to occur through trypsin-mediated cleavages, termed autolysis. Autolysis was also postulated to play a protective role against pancreatitis by eliminating prematurely activated intrapancreatic trypsin. However, autolysis of human cationic trypsin is very slow in vitro, which is inconsistent with the documented intestinal trypsin degradation or a putative protective role. Here we report that degradation of human cationic trypsin is triggered by chymotrypsin C, which selectively cleaves the Leu(81)-Glu(82) peptide bond within the Ca(2+) binding loop. Further degradation and inactivation of cationic trypsin is then achieved through tryptic cleavage of the Arg(122)-Val(123) peptide bond. Consequently, mutation of either Leu(81) or Arg(122) blocks chymotrypsin C-mediated trypsin degradation. Calcium affords protection against chymotrypsin C-mediated cleavage, with complete stabilization observed at 1 mM concentration. Chymotrypsin C is highly specific in promoting trypsin degradation, because chymotrypsin B1, chymotrypsin B2, elastase 2A, elastase 3A, or elastase 3B are ineffective. Chymotrypsin C also rapidly degrades all three human trypsinogen isoforms and appears identical to enzyme Y, the enigmatic trypsinogen-degrading activity described by Heinrich Rinderknecht in 1988. Taken together with previous observations, the results identify chymotrypsin C as a key regulator of activation and degradation of cationic trypsin. Thus, in the high Ca(2+) environment of the duodenum, chymotrypsin C facilitates trypsinogen activation, whereas in the lower intestines, chymotrypsin C promotes trypsin degradation as a function of decreasing luminal Ca(2+) concentrations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Guy O, Lombardo D, Bartelt DC, Amic J, Figarella C. Biochemistry. 1978;17:1669–1675. - PubMed
-
- Scheele G, Bartelt D, Bieger W. Gastroenterology. 1981;80:461–473. - PubMed
-
- Rinderknecht H, Renner IG, Abramson SB, Carmack C. Gastroenterology. 1984;86:681–692. - PubMed
-
- Rinderknecht H, Stace NH, Renner IG. Dig Dis Sci. 1985;30:65–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

