Myeloperoxidase: a target for new drug development?
- PMID: 17592500
- PMCID: PMC2078229
- DOI: 10.1038/sj.bjp.0707358
Myeloperoxidase: a target for new drug development?
Abstract
Myeloperoxidase (MPO), a member of the haem peroxidase-cyclooxygenase superfamily, is abundantly expressed in neutrophils and to a lesser extent in monocytes and certain type of macrophages. MPO participates in innate immune defence mechanism through formation of microbicidal reactive oxidants and diffusible radical species. A unique activity of MPO is its ability to use chloride as a cosubstrate with hydrogen peroxide to generate chlorinating oxidants such as hypochlorous acid, a potent antimicrobial agent. However, evidence has emerged that MPO-derived oxidants contribute to tissue damage and the initiation and propagation of acute and chronic vascular inflammatory disease. The fact that circulating levels of MPO have been shown to predict risks for major adverse cardiac events and that levels of MPO-derived chlorinated compounds are specific biomarkers for disease progression, has attracted considerable interest in the development of therapeutically useful MPO inhibitors. Today, detailed information on the structure of ferric MPO and its complexes with low- and high-spin ligands is available. This, together with a thorough understanding of reaction mechanisms including redox properties of intermediates, enables a rationale attempt in developing specific MPO inhibitors that still maintain MPO activity during host defence and bacterial killing but interfere with pathophysiologically persistent activation of MPO. The various approaches to inhibit enzyme activity of MPO and to ameliorate adverse effects of MPO-derived oxidants will be discussed. Emphasis will be put on mechanism-based inhibitors and high-throughput screening of compounds as well as the discussion of physiologically useful HOCl scavengers.
Figures


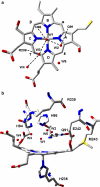
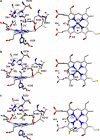
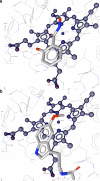
References
-
- Agner K. Verdoperoxidase. A ferment isolated from leukocytes. Acta Chem Scand A. 1941;2 Suppl. 8:1–62.
-
- Andrews PC, Krinsky NI. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J Biol Chem. 1982;257:13240–13245. - PubMed
-
- Arnhold J, Furtmüller PG, Regelsberger G, Obinger C. Redox properties of the couple compound I/native enzyme of human myeloperoxidase and eosinophil peroxidase. Eur J Biochem. 2001;268:5142–5148. - PubMed
-
- Arnhold J, Monzani E, Furtmüller PG, Zederbauer M, Casella L, Obinger C. Kinetics and thermodynamics of halide and nitrite oxidation by mammalian peroxidases. Eur J Inorg Chem. 2006;19:3801–3811.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

