State dependent dissociation of HERG channel inhibitors
- PMID: 17592502
- PMCID: PMC2189824
- DOI: 10.1038/sj.bjp.0707356
State dependent dissociation of HERG channel inhibitors
Abstract
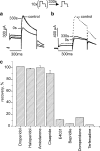
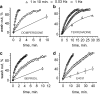
Background and purpose: Inhibition of HERG channels prolongs the ventricular action potential and the QT interval with the risk of torsade de pointes arrhythmias and sudden cardiac death. Many drugs induce greater inhibition of HERG channels when the cell membrane is depolarized frequently. The dependence of inhibition on the pulsing rate may yield different IC(50) values at different frequencies and thus affect the quantification of HERG channel block. We systematically compared the kinetics of HERG channel inhibition and recovery from block by 8 blockers at different frequencies.
Experimental approach: HERG channels were expressed heterologously in Xenopus oocytes and currents were measured with the two-electrode voltage clamp technique.
Key results: Frequency-dependent block was observed for amiodarone, cisapride, droperidol and haloperidol (group 1) whereas bepridil, domperidone, E-4031 and terfenadine (group 2) induced similar pulse-dependent block at all frequencies. With the group 1 compounds, HERG channels recovered from block in the presence of drug (recovery being voltage-dependent). No substantial recovery from block was observed with the second group of compounds. Washing out of bepridil, domperidone, E-4031 and terfenadine was substantially augmented by frequent pulsing. Mutation D540K in the HERG channel (which exhibits reopening at negative voltages) facilitated recovery from block by these compounds at -140 mV.
Conclusion and implications: Drug molecules dissociate at different rates from open and closed HERG channels ('use-dependent' dissociation). Our data suggest that apparently 'trapped' drugs (group 2) dissociated from the open channel state whereas group 1 compounds dissociated from open and resting states.
Figures
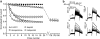

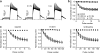


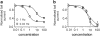
References
-
- Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992;262:809–817. - PubMed
-
- Choi SY, Koh YS, Jo SH. Inhibition of human ether-a-go-go-related gene K+ channel and IKr of guinea pig cardiomyocytes by antipsychotic drug trifluoperazine. J Pharmacol Exp Ther. 2005;313:888–895. - PubMed
-
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

