Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis
- PMID: 17592639
- PMCID: PMC1929064
- DOI: 10.1186/1471-2253-7-5
Drotrecogin alfa (activated) in severe sepsis: a systematic review and new cost-effectiveness analysis
Abstract
Background: Activated drotrecogin alfa (human activated protein C, rhAPC), is produced by recombinant DNA technology, and purports to improve clinical outcomes by counteracting the inflammatory and thrombotic consequences of severe sepsis. Controversy exists around the clinical benefits of this drug and an updated economic study that considers this variability is needed.
Methods: A systematic literature review was performed using Medline, Embase and the International Network of Agencies for Health Technology Assessment (INAHTA) databases to determine efficacy, safety and previous economic studies. Our economic model was populated with systematic estimates of these parameters and with population life tables for longer term survival information. Monte Carlo simulations were used to estimate the incremental cost-effectiveness ratios (ICERs) and variance for the decision analytic models.
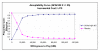
Results: Two randomized clinical trials (RCTS) of drotrecogin alfa in adults with severe sepsis and 8 previous economic studies were identified. Although associated with statistical heterogeneity, a pooled analysis of the RCTs did not show a statistically significant 28-day mortality benefit for drotrecogin alfa compared to placebo either for all patients (RR: 0.93, 95% CI: 0.69, 1.26) or those at highest risk as measured by APACHE II >or= 25 (RR: 0.90, 95% CI: 0.54, 1.49). Our economic analysis based on the totality of the available clinical evidence suggests that the cost-effectiveness of drotrecogin alfa is uncertain (< 59% probability that incremental cost-effectiveness ratio (ICER) life year gained (LYG) <or= $50,000/LYG) when applied to all patients with severe sepsis. The economic attractiveness of this therapy improves when administered to those at highest risk as assessed by APACHE II >or= 25 (93% probability ICER <or= $50,000/LYG) but these results are not robust to different measures of disease severity.
Conclusion: The evidence supporting the clinical and economic attractiveness of drotrecogin alfa is not conclusive and further research appears to be indicated.
Figures






References
-
- Food and Drug Administration: FDA Clinical Review. Drotrecogin alfa (activated) [Recombinant human activated protein C (rhAPC)] XIGRIS (TM) 2001. Parts 1 and 2. http://www.fda.gov/cder/biologics/review/droteli112101r1.pdf Last access: August 2006.
-
- United States Food and Drug Administration. Drotrecogin alfa (activated) letter of approval. http://www.fda.gov/cder/foi/appletter/2001/droteli112101L.pdf November 21st 2001. Last access: July 2006.
-
- European Medicines Agency. Xigris product information. http://www.emea.eu.int/humandocs/Humans/EPAR/xigris/Xigris.htm
-
- Health Canada. Notice of Compliance - Xigris - 31-01-2003. http://www.nocdatabase.ca/ Last access: July 2006.
LinkOut - more resources
Full Text Sources

