Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation
- PMID: 17594175
- PMCID: PMC1896221
- DOI: 10.1371/journal.pmed.0040230
Canine and human visual cortex intact and responsive despite early retinal blindness from RPE65 mutation
Abstract
Background: RPE65 is an essential molecule in the retinoid-visual cycle, and RPE65 gene mutations cause the congenital human blindness known as Leber congenital amaurosis (LCA). Somatic gene therapy delivered to the retina of blind dogs with an RPE65 mutation dramatically restores retinal physiology and has sparked international interest in human treatment trials for this incurable disease. An unanswered question is how the visual cortex responds after prolonged sensory deprivation from retinal dysfunction. We therefore studied the cortex of RPE65-mutant dogs before and after retinal gene therapy. Then, we inquired whether there is visual pathway integrity and responsivity in adult humans with LCA due to RPE65 mutations (RPE65-LCA).
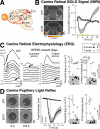
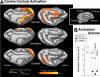
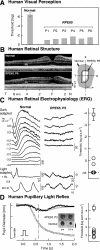
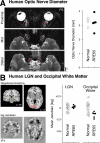
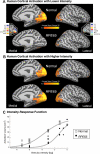
Methods and findings: RPE65-mutant dogs were studied with fMRI. Prior to therapy, retinal and subcortical responses to light were markedly diminished, and there were minimal cortical responses within the primary visual areas of the lateral gyrus (activation amplitude mean +/- standard deviation [SD] = 0.07% +/- 0.06% and volume = 1.3 +/- 0.6 cm(3)). Following therapy, retinal and subcortical response restoration was accompanied by increased amplitude (0.18% +/- 0.06%) and volume (8.2 +/- 0.8 cm(3)) of activation within the lateral gyrus (p < 0.005 for both). Cortical recovery occurred rapidly (within a month of treatment) and was persistent (as long as 2.5 y after treatment). Recovery was present even when treatment was provided as late as 1-4 y of age. Human RPE65-LCA patients (ages 18-23 y) were studied with structural magnetic resonance imaging. Optic nerve diameter (3.2 +/- 0.5 mm) was within the normal range (3.2 +/- 0.3 mm), and occipital cortical white matter density as judged by voxel-based morphometry was slightly but significantly altered (1.3 SD below control average, p = 0.005). Functional magnetic resonance imaging in human RPE65-LCA patients revealed cortical responses with a markedly diminished activation volume (8.8 +/- 1.2 cm(3)) compared to controls (29.7 +/- 8.3 cm(3), p < 0.001) when stimulated with lower intensity light. Unexpectedly, cortical response volume (41.2 +/- 11.1 cm(3)) was comparable to normal (48.8 +/- 3.1 cm(3), p = 0.2) with higher intensity light stimulation.
Conclusions: Visual cortical responses dramatically improve after retinal gene therapy in the canine model of RPE65-LCA. Human RPE65-LCA patients have preserved visual pathway anatomy and detectable cortical activation despite limited visual experience. Taken together, the results support the potential for human visual benefit from retinal therapies currently being aimed at restoring vision to the congenitally blind with genetic retinal disease.
Conflict of interest statement
Figures

References
-
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
-
- Batten ML, Imanishi Y, Tu DC, Doan T, Zhu L, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. - DOI - PMC - PubMed
-
- Pawlyk BS, Smith AJ, Buch PK, Adamian M, Hong DH, et al. Gene replacement therapy rescues photoreceptor degeneration in a murine model of Leber congenital amaurosis lacking RPGRIP. Invest Ophthalmol Vis Sci. 2005;46:3039–3045. - PubMed
-
- Williams ML, Coleman JE, Haire SE, Aleman TS, Cideciyan AV, et al. Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med. 2006;3:e201. doi: 10.1371/journal.pmed.0030201. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY13729/EY/NEI NIH HHS/United States
- U10 EY017280/EY/NEI NIH HHS/United States
- EY10016/EY/NEI NIH HHS/United States
- R01 EY006855/EY/NEI NIH HHS/United States
- EY06855/EY/NEI NIH HHS/United States
- R01 EY013132/EY/NEI NIH HHS/United States
- U10 EY013729/EY/NEI NIH HHS/United States
- EY17280/EY/NEI NIH HHS/United States
- P30 EY001583/EY/NEI NIH HHS/United States
- Wellcome Trust/United Kingdom
- P30 NS045839/NS/NINDS NIH HHS/United States
- R01 EY010016/EY/NEI NIH HHS/United States
- EY13132/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

