Biomimetic approach to cardiac tissue engineering
- PMID: 17594967
- PMCID: PMC2440401
- DOI: 10.1098/rstb.2007.2121
Biomimetic approach to cardiac tissue engineering
Abstract
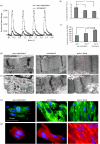
Here, we review an approach to tissue engineering of functional myocardium that is biomimetic in nature, as it involves the use of culture systems designed to recapitulate some aspects of the actual in vivo environment. To mimic the capillary network, subpopulations of neonatal rat heart cells were cultured on a highly porous elastomer scaffold with a parallel array of channels perfused with culture medium. To mimic oxygen supply by haemoglobin, the culture medium was supplemented with a perfluorocarbon (PFC) emulsion. Constructs cultivated in the presence of PFC contained higher amounts of DNA and cardiac markers and had significantly better contractile properties than control constructs cultured without PFC. To induce synchronous contractions of cultured constructs, electrical signals mimicking those in native heart were applied. Over only 8 days of cultivation, electrical stimulation induced cell alignment and coupling, markedly increased the amplitude of synchronous construct contractions and resulted in a remarkable level of ultrastructural organization. The biomimetic approach is discussed in the overall context of cardiac tissue engineering, and the possibility to engineer functional human cardiac grafts based on human stem cells.
Figures





References
-
- Akins R.E, Boyce R.A, Madonna M.L, Schroedl N.A, Gonda S.R, McLaughlin T.A, Hartzell C.R. Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng. 1999;5:103–118. doi:10.1089/ten.1999.5.103 - DOI - PubMed
-
- Balsam L.B, Wagers A.J, Christensen J.L, Kofidis T, Weissman I.L, Robbins R.C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi:10.1038/nature02460 - DOI - PubMed
-
- Beltrami A.P, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi:10.1016/S0092-8674(03)00687-1 - DOI - PubMed
-
- Brilla C.G, Maisch B, Rupp H, Sunck R, Zhou G, Weber K.T. Pharmacological modulation of cardiac fibroblast function. Herz. 1995;20:127–135. - PubMed
-
- Burlew B.S, Weber K.T. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–98. doi:10.1007/s00059-002-2354-y - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
