Total synthesis of leucascandrolide a: a new application of the Mukaiyama aldol-Prins reaction
- PMID: 17595145
- PMCID: PMC2504509
- DOI: 10.1021/jo070901r
Total synthesis of leucascandrolide a: a new application of the Mukaiyama aldol-Prins reaction
Abstract
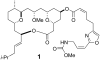
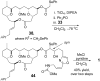
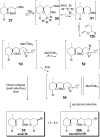
A total synthesis of the marine natural product leucascandrolide A has been completed. The titanium tetrabromide-mediated Mukaiyama aldol-Prins (MAP) reaction with aldehydes developed in our group provided a highly convergent and stereoselective method for assembling the core of the molecule. A new class of MAP reactions with acetals is introduced, and mechanistic considerations for both MAP methods are described. The total synthesis was completed by coupling of the side chain through two avenues: a known Still-Gennari olefination and a new Z-selective aldol/dehydroselenation reaction. Both procedures were highly selective and provided the natural product.
Figures







Similar articles
-
[4 + 2]-annulations of chiral organosilanes: Application to the total synthesis of leucascandrolide A.J Org Chem. 2007 Jan 5;72(1):2-24. doi: 10.1021/jo0610412. J Org Chem. 2007. PMID: 17194076
-
Mukaiyama aldol-Prins cyclization cascade reaction: a formal total synthesis of leucascandrolide A.J Am Chem Soc. 2001 Aug 29;123(34):8420-1. doi: 10.1021/ja011377n. J Am Chem Soc. 2001. PMID: 11516301 No abstract available.
-
Synthetic efforts toward the macrolactone core of Leucascandrolide A.J Org Chem. 2008 Mar 7;73(5):1864-80. doi: 10.1021/jo701315h. Epub 2008 Jan 30. J Org Chem. 2008. PMID: 18229938
-
The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis.Nat Prod Rep. 2014 Apr;31(4):563-94. doi: 10.1039/c3np70102f. Epub 2014 Mar 5. Nat Prod Rep. 2014. PMID: 24595879 Review.
-
Very Recent Advances in Vinylogous Mukaiyama Aldol Reactions and Their Applications to Synthesis.Molecules. 2019 Aug 22;24(17):3040. doi: 10.3390/molecules24173040. Molecules. 2019. PMID: 31443344 Free PMC article. Review.
Cited by
-
Synthesis of (-)-okilactomycin by a Prins-type fragment-assembly strategy.Angew Chem Int Ed Engl. 2011 Jun 20;50(26):5892-5. doi: 10.1002/anie.201102037. Epub 2011 May 10. Angew Chem Int Ed Engl. 2011. PMID: 21560215 Free PMC article. No abstract available.
-
Catalyst-controlled stereoselective olefin metathesis as a principal strategy in multistep synthesis design: a concise route to (+)-neopeltolide.Angew Chem Int Ed Engl. 2015 Jan 2;54(1):215-20. doi: 10.1002/anie.201409120. Epub 2014 Nov 6. Angew Chem Int Ed Engl. 2015. PMID: 25377347 Free PMC article.
-
Prins cyclization-mediated stereoselective synthesis of tetrahydropyrans and dihydropyrans: an inspection of twenty years.Beilstein J Org Chem. 2021 Apr 29;17:932-963. doi: 10.3762/bjoc.17.77. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33981366 Free PMC article. Review.
-
Lewis Acid-Promoted Mukaiyama Aldol-Prins (MAP) Cyclizations of Acetals, Ketals, α-Acetoxy Ethers, and Orthoformates.Synlett. 2008 Feb 12;2008(3):363-366. doi: 10.1055/s-2008-1032053. Synlett. 2008. PMID: 20936058 Free PMC article.
-
Mechanistic Analysis of Oxidative C-H Cleavages Using Inter- and Intramolecular Kinetic Isotope Effects.Tetrahedron. 2009 Dec 26;65(52):10830-10836. doi: 10.1016/j.tet.2009.10.088. Tetrahedron. 2009. PMID: 20640173 Free PMC article.
References
-
- D'Ambrosio M, Guerriero A, Debitus C, Pietra F. Helv Chim Acta. 1996;79:51–60.
-
-
This particular class of sponges typically produces compounds consisting of 2-amino imidazoles. See reference .
-
-
- Hornberger KR, Hamblett CL, Leighton JL. J Am Chem Soc. 2000;122:12894–12895.
-
- Paterson I, Tudge M. Angew Chem, Int Ed. 2003;42:343–347. - PubMed
- Paterson I, Tudge M. Tetrahedron. 2003;59:6833–6849.
-
- Wang Y, Janjic J, Kozmin SA. Pure Appl Chem. 2005;77:1161–1169.
- Wang Y, Janjic J, Kozmin SA. J Am Chem Soc. 2002;124:13670–13671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

