Assessment of N-glycan heterogeneity of cactus glycoproteins by one-dimensional gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- PMID: 17595312
- PMCID: PMC2062550
Assessment of N-glycan heterogeneity of cactus glycoproteins by one-dimensional gel electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
Abstract
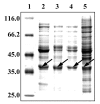
Artificial environmental conditions in tissue culture, such as elevated relative humidity and rich nutrient medium, can influence and modify tissue growth and induce spontaneous changes from characteristic organization pattern to unorganized callus. As succulent plants with crassulacean acid metabolism, cacti are particularly susceptible to this altered growth environment. Glycosylated proteins of Mammillaria gracillis tissues cultivated in vitro, separated by SDS-PAGE, were detected with Con A after the transfer of proteins onto the nitrocellulose membrane. The glycan components were further characterized by affinity blotting with different lectins (GNA, DSA, PNA, and RCA(120)). The results revealed significant differences in glycoprotein pattern among the investigated cactus tissues (shoot, callus, hyperhydric regenerant, and tumor). To test whether the N-glycosylation of the same protein can vary in different developmental stages of cactus tissue, the N-glycans were analyzed by MALDI-TOF MS after in-gel deglycosylation of the excised 38-kDa protein band. Paucimannosidic-type N-glycans were detected in oligosaccharide mixtures from shoot and callus, while the hyperhydric regenerant and tumor shared glycans of complex type. The hybrid oligosaccharide structures were found only in tumor tissue. These results indicate that the adaptation of plant cells to artificial environment in tissue culture is reflected in N-glycosylation, and structures of N-linked glycans vary with different developmental stages of Mammillaria gracillis tissues.
Figures




Similar articles
-
Glycoproteomic survey of Mammillaria gracillis tissues grown in vitro.J Proteome Res. 2006 Jul;5(7):1658-66. doi: 10.1021/pr0600327. J Proteome Res. 2006. PMID: 16823973
-
Glycoproteomics of N-glycosylation by in-gel deglycosylation and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry mapping: application to congenital disorders of glycosylation.Proteomics. 2005 Jul;5(10):2689-701. doi: 10.1002/pmic.200401312. Proteomics. 2005. PMID: 15912511
-
Structural determination of N-linked carbohydrates by matrix-assisted laser desorption/ionization-mass spectrometry following enzymatic release within sodium dodecyl sulphate-polyacrylamide electrophoresis gels: application to species-specific glycosylation of alpha1-acid glycoprotein.Electrophoresis. 1998 Aug;19(11):1950-9. doi: 10.1002/elps.1150191113. Electrophoresis. 1998. PMID: 9740055
-
Methods in enzymology: O-glycosylation of proteins.Methods Enzymol. 2005;405:139-71. doi: 10.1016/S0076-6879(05)05007-X. Methods Enzymol. 2005. PMID: 16413314 Review.
-
Analysis of N- and O-linked glycans from glycoproteins using MALDI-TOF mass spectrometry.Methods Mol Biol. 2009;534:5-21. doi: 10.1007/978-1-59745-022-5_1. Methods Mol Biol. 2009. PMID: 19277556 Review.
References
-
- Elias-Rocha MA, Santos-Diaz MD, Arredondo-Gomez A. Propagation of Mammillaria candida (Cactaceae) by tissue culture techniques. Haseltonia. 1998;6:96–101.
-
- Krsnik-Rasol M, Balen B. Electrophoretic protein patterns and peroxidase activity related to morphogenesis in Mammillaria gracillis tissue culture. Acta Bot Croat. 2001;60:219–226.
-
- Gaspar T. The concept of cancer in in vitro plant cultures and the implication of habituation to hormones and hyperhydricity. Plant Tissue Cult Biotechnol. 1995;1:126–136.
-
- Gaspar T, Kevers C, Bisbis B, et al. Loss of plant organogenic totipotency in the course of in vitro neoplastic progression. In Vitro Cell Dev Biol-Plant. 2000;36:171–181.
-
- Krsnik-Rasol M, Jelaska S, Šerman D. Isoperoxidase—early indicators of somatic embryoid differentiation in pumpkin tissue. Acta Bot Croat. 1982;41:33–39.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
