Comparison of marker gene expression in chondrocytes from patients receiving autologous chondrocyte transplantation versus osteoarthritis patients
- PMID: 17596264
- PMCID: PMC2206334
- DOI: 10.1186/ar2218
Comparison of marker gene expression in chondrocytes from patients receiving autologous chondrocyte transplantation versus osteoarthritis patients
Abstract
Currently, autologous chondrocyte transplantation (ACT) is used to treat traumatic cartilage damage or osteochondrosis dissecans, but not degenerative arthritis. Since substantial refinements in the isolation, expansion and transplantation of chondrocytes have been made in recent years, the treatment of early stage osteoarthritic lesions using ACT might now be feasible. In this study, we determined the gene expression patterns of osteoarthritic (OA) chondrocytes ex vivo after primary culture and subculture and compared these with healthy chondrocytes ex vivo and with articular chondrocytes expanded for treatment of patients by ACT. Gene expression profiles were determined using quantitative RT-PCR for type I, II and X collagen, aggrecan, IL-1beta and activin-like kinase-1. Furthermore, we tested the capability of osteoarthritic chondrocytes to generate hyaline-like cartilage by implanting chondrocyte-seeded collagen scaffolds into immunodeficient (SCID) mice. OA chondrocytes ex vivo showed highly elevated levels of IL-1beta mRNA, but type I and II collagen levels were comparable to those of healthy chondrocytes. After primary culture, IL-1beta levels decreased to baseline levels, while the type II and type I collagen mRNA levels matched those found in chondrocytes used for ACT. OA chondrocytes generated type II collagen and proteoglycan-rich cartilage transplants in SCID mice. We conclude that after expansion under suitable conditions, the cartilage of OA patients contains cells that are not significantly different from those from healthy donors prepared for ACT. OA chondrocytes are also capable of producing a cartilage-like tissue in the in vivo SCID mouse model. Thus, such chondrocytes seem to fulfil the prerequisites for use in ACT treatment.
Figures

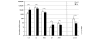
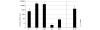

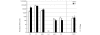
References
-
- Hunter W. Of the structures and diseases of articular cartilages. 1743. Clin Orthop Relat Res. 1995;317:3–6. - PubMed
-
- Marlovits S, Hombauer M, Tamandl D, Vecsei V, Schlegel W. Quantitative analysis of gene expression in human articular chondrocytes in monolayer culture. Int J Molec Med. 2004;13:281–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

