De novo protein synthesis is required for lytic cycle reactivation of Epstein-Barr virus, but not Kaposi's sarcoma-associated herpesvirus, in response to histone deacetylase inhibitors and protein kinase C agonists
- PMID: 17596302
- PMCID: PMC1951462
- DOI: 10.1128/JVI.00982-07
De novo protein synthesis is required for lytic cycle reactivation of Epstein-Barr virus, but not Kaposi's sarcoma-associated herpesvirus, in response to histone deacetylase inhibitors and protein kinase C agonists
Abstract
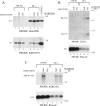
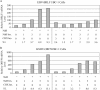
The oncogenic human gammaherpesviruses, Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), are latent in cultured lymphoma cells. We asked whether reactivation from latency of either virus requires de novo protein synthesis. Using Northern blotting and quantitative reverse transcriptase PCR, we measured the kinetics of expression of the lytic cycle activator genes and determined whether abundance of mRNAs encoding these genes from either virus was reduced by treatment with cycloheximide (CHX), an inhibitor of protein synthesis. CHX blocked expression of mRNAs of EBV BZLF1 and BRLF1, the two EBV lytic cycle activator genes, when HH514-16 Burkitt lymphoma cells were treated with histone deacetylase (HDAC) inhibitors, sodium butyrate or trichostatin A, or a DNA methyltransferase inhibitor, 5-Aza-2'-deoxycytidine. CHX also inhibited EBV lytic cycle activation in B95-8 marmoset lymphoblastoid cells by phorbol ester phorbol-12-myristate-13-acetate (TPA). EBV lytic cycle induction became resistant to CHX between 4 and 6 h after application of the inducing stimulus. KSHV lytic cycle activation, as assessed by ORF50 mRNA expression, was rapidly induced by the HDAC inhibitors, sodium butyrate and trichostatin A, in HH-B2 primary effusion lymphoma cells. In HH-B2 cells, CHX did not inhibit, but enhanced, expression of the KSHV lytic cycle activator gene, ORF50. In BC-1, a primary effusion lymphoma cell line that is dually infected with EBV and KSHV, CHX blocked EBV BRLF1 lytic gene expression induced by TPA and sodium butyrate; KSHV ORF50 mRNA induced simultaneously in the same cells by the same inducing stimuli was resistant to CHX. The experiments show, for the cell lines and inducing agents studied, that the EBV BZLF1 and BRLF1 genes do not behave with "immediate-early" kinetics upon reactivation from latency. KSHV ORF50 is a true "immediate-early" gene. Our results indicate that the mechanism by which HDAC inhibitors and TPA induce lytic cycle gene expression of the two viruses differs and suggest that EBV but not KSHV requires one or more proteins to be newly synthesized between 4 and 6 h after application of an inducing stimulus.
Figures






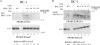
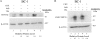
References
-
- Bartkiewicz, M., H. Gold, and S. Altman. 1989. Identification and characterization of an RNA molecule that copurifies with RNase P activity from HeLa cells. Genes Dev. 3:488-499. - PubMed
-
- Beelman, C. A., and R. Parker. 1994. Differential effects of translational inhibition in cis and in trans on the decay of the unstable yeast MFA2 mRNA. J. Biol. Chem. 269:9687-9692. - PubMed
-
- Ben-Sasson, S. A., and G. Klein. 1981. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int. J. Cancer 28:131-135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

