A rapid progressor-specific variant clone of simian immunodeficiency virus replicates efficiently in vivo only in the absence of immune responses
- PMID: 17596304
- PMCID: PMC1951398
- DOI: 10.1128/JVI.00614-07
A rapid progressor-specific variant clone of simian immunodeficiency virus replicates efficiently in vivo only in the absence of immune responses
Abstract
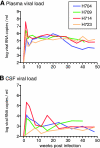
A subset of simian immunodeficiency virus (SIV)-infected macaques progresses rapidly to disease with transient SIV-specific immune responses and high viral loads. Unique SIV variants with convergent Env mutations evolve in these rapid progressor (RP) macaques. To address the pathogenic significance of RP-specific variants, we generated infectious molecular clones from the terminal-phase plasma of an RP macaque. Inoculation of macaques with a representative clone, SIVsmH635FC, resulted in a persistent viremia, comparable to that produced by pathogenic SIVsmE543-3, and a chronic disease with progressive loss of CD4(+) T cells. However, SIVsmH635FC did not reproduce the rapid-disease phenomenon. Molecular analyses of viruses from these macaques revealed rapid reversion to the wild-type SIVsmE543-3 sequence at two RP-specific sites and slower reversion at another three sites. SIVsmH635FC infection was not sufficient to cause rapid progression even following coinoculation with SIVsmE543-3, despite acute depletion of memory CD4(+) T cells. SIVsmH635FC competed efficiently during primary infection in the coinoculated macaques, but SIVsmE543-3 predominated after the development of SIV-specific immune responses. These data suggest that the replication fitness of the RP variant was similar to that of SIVsmE543-3 in a naïve host; however, SIVsmH635FC was at a disadvantage following the development of SIV-specific immune responses. Consistent with these findings, neutralization assays revealed that SIVsmH635FC was highly sensitive to neutralization but that the parental SIVsmE543-3 strain was highly resistant. This study suggests that the evolution of RP-specific variants is the result of replication in a severely immunocompromised host, rather than the direct cause of rapid progression.
Figures




References
-
- Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. - PubMed
-
- Brown, C. R., M. Czapiga, J. Kabat, Q. Dang, I. Ourmanov, Y. Nishimura, M. A. Martin, and V. M. Hirsch. 2007. Unique pathology in simian immunodeficiency virus-infected rapid-progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J. Virol. 81:5594-5606. - PMC - PubMed
-
- Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

