The RNA binding domain of influenza A virus NS1 protein affects secretion of tumor necrosis factor alpha, interleukin-6, and interferon in primary murine tracheal epithelial cells
- PMID: 17596305
- PMCID: PMC1951395
- DOI: 10.1128/JVI.00989-07
The RNA binding domain of influenza A virus NS1 protein affects secretion of tumor necrosis factor alpha, interleukin-6, and interferon in primary murine tracheal epithelial cells
Erratum in
- J Virol. 2007 Nov;81(22):12717
Abstract
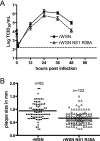
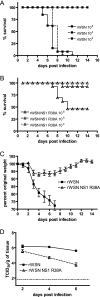
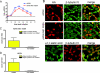
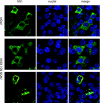
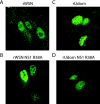
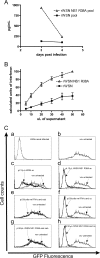
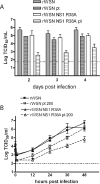
Primary differentiated respiratory epithelial cell cultures closely model the in vivo environment and allow for studies of innate immune responses generated specifically by epithelial cells, the primary cell type infected by human influenza A virus strains. We used primary murine tracheal epithelial cell (mTEC) cultures to investigate antiviral and cytokine responses to influenza A virus infection, focusing on the contribution of the RNA binding domain of the NS1 protein. rWSN NS1 R38A replication is attenuated in mTEC cultures; however, viral antigen is detected predominantly in ciliated cells, similar to wild-type virus. NS1 and NS1 R38A proteins display a primarily cytoplasmic localization in infected mTEC cultures. Increased production of tumor necrosis factor alpha, interleukin-6, and beta interferon is observed during rWSN NS1 R38A infection, and cytokines are secreted in a directional manner. Cytokine pretreatment of mTEC cultures and Vero cells suggest that rWSN NS1 R38A is more sensitive to the presence of antiviral/inflammatory cytokines than wild-type virus. Our results demonstrate that the RNA binding domain is a critical regulator of both cytokine production and cytokine sensitivity during influenza A virus infection of primary tracheal epithelial cells.
Figures
References
-
- Alonso-Caplen, F. V., M. E. Nemeroff, Y. Qiu, and R. M. Krug. 1992. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 6:255-267. - PubMed
-
- Anonymous. 2004. Update: influenza-associated deaths reported among children aged <18 years—United States, 2003-04 influenza season. Ann. Pharmacother. 38:367-368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

