Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro
- PMID: 17596307
- PMCID: PMC1951389
- DOI: 10.1128/JVI.00827-07
Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro
Abstract
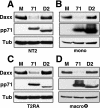
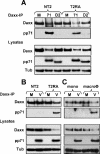
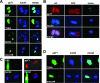
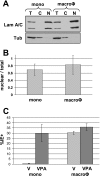
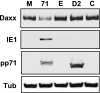
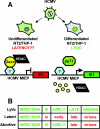
In addition to productive lytic infections, herpesviruses such as human cytomegalovirus (HCMV) establish a reservoir of latently infected cells that permit lifelong colonization of the host. When latency is established, the viral immediate-early (IE) genes that initiate the lytic replication cycle are not expressed. HCMV IE gene expression at the start of a lytic infection is facilitated by the viral pp71 protein, which is delivered to cells by infectious viral particles. pp71 neutralizes the Daxx-mediated cellular intrinsic immune defense that silences IE gene expression by generating a repressive chromatin structure on the viral major IE promoter (MIEP). In naturally latently infected cells and in cells latently infected in vitro, the MIEP also adopts a similar silenced chromatin structure. Here we analyze the role of Daxx in quiescent HCMV infections in vitro that mimic some, but not all, of the characteristics of natural latency. We show that in these "latent-like" infections, the Daxx-mediated defense that represses viral gene expression is not disabled because pp71 and Daxx localize to different cellular compartments. We demonstrate that Daxx is required to establish quiescent HCMV infections in vitro because in cells that would normally foster the establishment of these latent-like infections, the loss of Daxx causes the lytic replication cycle to be initiated. Importantly, the lytic cycle is inefficiently completed, which results in an abortive infection. Our work demonstrates that, in certain cell types, HCMV must silence its own gene expression to establish quiescence and prevent abortive infection and that the virus usurps a Daxx-mediated cellular intrinsic immune defense mechanism to do so. This identifies Daxx as one of the likely multiple viral and cellular determinants in the pathway of HCMV quiescence in vitro, and perhaps in natural latent infections as well.
Figures


References
-
- Albrecht, T., M. Nachtigal, S. C. St. Jeor, and F. Rapp. 1976. Induction of cellular DNA synthesis and increased mitotic activity in Syrian hamster embryo cells abortively infected with human cytomegalovirus. J. Gen. Virol. 30:167-177. - PubMed
-
- Andrews, P. W., I. Damjanov, D. Simon, G. S. Banting, C. Carlin, N. C. Dracopoli, and J. Fogh. 1984. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab. Investig. 50:147-162. - PubMed
-
- Becker, K. A., L. Florin, C. Sapp, and M. Sapp. 2003. Dissection of human papillomavirus type 33 L2 domains involved in nuclear domains (ND) 10 homing and reorganization. Virology 314:161-167. - PubMed
-
- Bego, M. G., and S. St. Jeor. 2006. Human cytomegalovirus infection of cells of hematopoietic origin: HCMV-induced immunosuppression, immune evasion, and latency. Exp. Hematol. 34:555-570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

