A baculovirus-expressed dicistrovirus that is infectious to aphids
- PMID: 17596314
- PMCID: PMC1951450
- DOI: 10.1128/JVI.00417-07
A baculovirus-expressed dicistrovirus that is infectious to aphids
Erratum in
-
A baculovirus-expressed dicistrovirus that is infectious to aphids.J Virol. 2014 Mar;88(6):3610. doi: 10.1128/JVI.03821-13. J Virol. 2014. PMID: 24567350 Free PMC article. No abstract available.
Abstract
Detailed investigation of virus replication is facilitated by the construction of a full-length infectious clone of the viral genome. To date, this has not been achieved for members of the family Dicistroviridae. Here we demonstrate the construction of a baculovirus that expresses a dicistrovirus that is infectious in its natural host. We inserted a full-length cDNA clone of the genomic RNA of the dicistrovirus Rhopalosiphum padi virus (RhPV) into a baculovirus expression vector. Virus particles containing RhPV RNA accumulated in the nuclei of baculovirus-infected Sf21 cells expressing the recombinant RhPV clone. These virus particles were infectious in R. padi, a ubiquitous aphid vector of major cereal viruses. The recombinant virus was transmitted efficiently between aphids, despite the presence of 119 and 210 vector-derived bases that were stably maintained at the 5' and 3' ends, respectively, of the RhPV genome. The maintenance of such a nonviral sequence was surprising considering that most RNA viruses tolerate few nonviral bases beyond their natural termini. The use of a baculovirus to express a small RNA virus opens avenues for investigating replication of dicistroviruses and may allow large-scale production of these viruses for use as biopesticides.
Figures

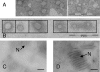

Similar articles
-
Infectious genomic RNA of Rhopalosiphum padi virus transcribed in vitro from a full-length cDNA clone.Virology. 2008 Jun 5;375(2):401-11. doi: 10.1016/j.virol.2008.02.008. Epub 2008 Mar 12. Virology. 2008. PMID: 18339417
-
Viral metagenomics of aphids present in bean and maize plots on mixed-use farms in Kenya reveals the presence of three dicistroviruses including a novel Big Sioux River virus-like dicistrovirus.Virol J. 2017 Oct 2;14(1):188. doi: 10.1186/s12985-017-0854-x. Virol J. 2017. PMID: 28969654 Free PMC article.
-
The Rhopalosiphum padi virus 5' internal ribosome entry site is functional in Spodoptera frugiperda 21 cells and in their cell-free lysates: implications for the baculovirus expression system.J Gen Virol. 2004 Jun;85(Pt 6):1565-1569. doi: 10.1099/vir.0.79992-0. J Gen Virol. 2004. PMID: 15166440
-
Optimizing the baculovirus expression vector system.Methods. 2011 Sep;55(1):52-7. doi: 10.1016/j.ymeth.2011.06.011. Epub 2011 Sep 13. Methods. 2011. PMID: 21945427 Review.
-
Dicistroviruses.Annu Rev Entomol. 2010;55:129-50. doi: 10.1146/annurev-ento-112408-085457. Annu Rev Entomol. 2010. PMID: 19961327 Review.
Cited by
-
Baculovirus expression of cloned porcine arterivirus generates infectious particles in both insect and mammalian cells.J Biotechnol. 2010 Oct 15;150(2):251-8. doi: 10.1016/j.jbiotec.2010.08.009. Epub 2010 Aug 20. J Biotechnol. 2010. PMID: 20728481 Free PMC article.
-
Toxins for transgenic resistance to hemipteran pests.Toxins (Basel). 2012 Jun;4(6):405-29. doi: 10.3390/toxins4060405. Epub 2012 Jun 4. Toxins (Basel). 2012. PMID: 22822455 Free PMC article. Review.
References
-
- Agrawal, D. K., and J. E. Johnson. 1995. Assembly of the T = 4 Nudaurelia capensis omega virus capsid protein, post-translational cleavage, and specific encapsidation of its mRNA in a baculovirus expression system. Virology 207:89-97. - PubMed
-
- Benjeddou, M., N. Leat, M. Allsopp, and S. Davison. 2002. Development of infectious transcripts and genome manipulation of Black queen-cell virus of honey bees. J. Gen. Virol. 83:3139-3146. - PubMed
-
- Black, B. C., L. A. Brennan, P. M. Dierks, and I. E. Gard. 1997. Commercialization of baculoviral insecticides, p. 341-388. In L. K. Miller (ed.), The baculoviruses. Plenum Press, New York, NY.
-
- Boyapalle, S., N. Pal, W. A. Miller, and B. C. Bonning. 2007. A glassy-winged sharpshooter cell line supports replication of Rhopalosiphum padi virus (Dicistroviridae). J. Invertebr. Pathol. 94:130-139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

