An adenovirus vector-mediated reverse genetics system for influenza A virus generation
- PMID: 17596315
- PMCID: PMC1951417
- DOI: 10.1128/JVI.01042-07
An adenovirus vector-mediated reverse genetics system for influenza A virus generation
Abstract
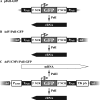
Plasmid-based reverse genetics systems allow the generation of influenza A virus entirely from cloned cDNA. However, since the efficiency of virus generation is dependent on the plasmid transfection efficiency of cells, virus generation is difficult in cells approved for vaccine production that have low transfection efficiencies (e.g., Vero cells). Here we established an alternative reverse genetics system for influenza virus generation by using an adenovirus vector (AdV) which achieves highly efficient gene transfer independent of cell transfection efficiency. This AdV-mediated reverse genetics system will be useful for generating vaccine seed strains and for basic influenza virus studies.
Figures

Similar articles
-
Efficient generation and growth of influenza virus A/PR/8/34 from eight cDNA fragments.Virus Res. 2004 Jul;103(1-2):155-61. doi: 10.1016/j.virusres.2004.02.028. Virus Res. 2004. PMID: 15163504
-
Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system.Vaccine. 2005 Apr 22;23(22):2943-52. doi: 10.1016/j.vaccine.2004.08.054. Vaccine. 2005. PMID: 15780743
-
An improved reverse genetics system for influenza A virus generation and its implications for vaccine production.Proc Natl Acad Sci U S A. 2005 Nov 15;102(46):16825-9. doi: 10.1073/pnas.0505587102. Epub 2005 Nov 2. Proc Natl Acad Sci U S A. 2005. PMID: 16267134 Free PMC article.
-
From the first to the third generation adenoviral vector: what parameters are governing the production yield?Biotechnol Adv. 2009 Mar-Apr;27(2):133-44. doi: 10.1016/j.biotechadv.2008.10.003. Epub 2008 Oct 31. Biotechnol Adv. 2009. PMID: 19013226 Review.
-
Lentiviral vectors: are they the future of animal transgenesis?Physiol Genomics. 2007 Oct 22;31(2):159-73. doi: 10.1152/physiolgenomics.00069.2007. Epub 2007 Aug 7. Physiol Genomics. 2007. PMID: 17684037 Review.
Cited by
-
Adenovirus vector-mediated assay system for hepatitis C virus replication.Nucleic Acids Res. 2011 May;39(10):e64. doi: 10.1093/nar/gkr047. Epub 2011 Feb 8. Nucleic Acids Res. 2011. PMID: 21306994 Free PMC article.
-
A cell-based screening system for influenza A viral RNA transcription/replication inhibitors.Sci Rep. 2013;3:1106. doi: 10.1038/srep01106. Epub 2013 Jan 22. Sci Rep. 2013. PMID: 23346363 Free PMC article.
-
Enhanced growth of influenza vaccine seed viruses in vero cells mediated by broadening the optimal pH range for virus membrane fusion.J Virol. 2012 Feb;86(3):1405-10. doi: 10.1128/JVI.06009-11. Epub 2011 Nov 16. J Virol. 2012. PMID: 22090129 Free PMC article.
-
Influenza Reverse Genetics-Historical Perspective.Cold Spring Harb Perspect Med. 2021 Apr 1;11(4):a038547. doi: 10.1101/cshperspect.a038547. Cold Spring Harb Perspect Med. 2021. PMID: 31964649 Free PMC article. Review.
-
Many ways to make an influenza virus--review of influenza virus reverse genetics methods.Influenza Other Respir Viruses. 2013 May;7(3):249-56. doi: 10.1111/j.1750-2659.2012.00392.x. Epub 2012 Jun 19. Influenza Other Respir Viruses. 2013. PMID: 22712782 Free PMC article. Review.
References
-
- Goto, H., R. C. Bethell, and Y. Kawaoka. 1997. Mutations affecting the sensitivity of the influenza virus neuraminidase to 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid. Virology 238:265-272. - PubMed
-
- Hoffmann, E., G. Neumann, G. Hobom, R. G. Webster, and Y. Kawaoka. 2000. “Ambisense” approach for the generation of influenza A virus: vRNA and mRNA synthesis from one template. Virology 267:310-317. - PubMed
-
- Horimoto, T., A. Takada, K. Fujii, H. Goto, M. Hatta, S. Watanabe, K. Iwatsuki-Horimoto, M. Ito, Y. Tagawa-Sakai, S. Yamada, H. Ito, T. Ito, M. Imai, S. Itamura, T. Odagiri, M. Tashiro, W. Lim, Y. Guan, M. Peiris, and Y. Kawaoka. 2006. The development and characterization of H5 influenza virus vaccines derived from a 2003 human isolate. Vaccine 24:3669-3676. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

