PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors
- PMID: 17596331
- PMCID: PMC2258221
- DOI: 10.1152/ajpregu.00337.2007
PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors
Abstract
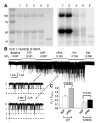
ATP-sensitive K(+) (K(ATP)) channels are activated by several vasodilating hormones and neurotransmitters through the PKA pathway. Here, we show that phosphorylation at Ser1387 of the SUR2B subunit is critical for the channel activation. Experiments were performed in human embryonic kidney (HEK) 293 cells expressing the cloned Kir6.1/SUR2B channel. In whole cell patch, the Kir6.1/SUR2B channel activity was stimulated by isoproterenol via activation of beta(2) receptors. This effect was blocked in the presence of inhibitors for adenylyl cyclase or PKA. Similar channel activation was seen by exposing inside-out patches to the catalytic subunit of PKA. Because none of the previously suggested PKA phosphorylation sites accounted for the channel activation, we performed systematic mutational analysis on Kir6.1 and SUR2B. Two serine residues (Ser1351, Ser1387) located in the NBD2 of SUR2B were critical for the channel activation. In vitro phosphorylation experiments showed that Ser1387 but not Ser1351 was phosphorylated by PKA. The PKA-dependent activation of cell-endogenous K(ATP) channels was observed in acutely dissociated mesenteric smooth myocytes and isolated mesenteric artery rings, where activation of these channels contributed significantly to the isoproterenol-induced vasodilation. Taken together, these results indicate that the Kir6.1/SUR2B channel is a target of beta(2) receptors and that the channel activation relies on PKA phosphorylation of SUR2B at Ser1387.
Figures


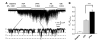




References
-
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 1999;20:101–135. - PubMed
-
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. - PubMed
-
- Cao K, Tang GH, Hu DH, Wang R. Molecular basis of ATP-sensitive K+ channels in rat vascular smooth muscles. Biochem Biophys Res Commun. 2002;296:463–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

