Comparison of the abbott 7000 and bayer 340 systems for measurement of hepatitis C virus load
- PMID: 17596352
- PMCID: PMC2045268
- DOI: 10.1128/JCM.00202-07
Comparison of the abbott 7000 and bayer 340 systems for measurement of hepatitis C virus load
Abstract
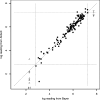
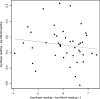
This study compared two commercially available assays for the measurement of hepatitis C virus (HCV) RNA levels, the Bayer HCV RNA (version 3.0) branched DNA assay and the Abbott HCV analyte-specific reagent real-time PCR assay, to assess their quantitative relationships, ease of performance, and time to completion. The study group consisted of randomly selected patients from the NIAID human immunodeficiency virus (HIV) outpatient clinic who were infected with HIV type 1 and HCV. One hundred eighty-four samples from 66 patients coinfected with HIV and HCV receiving treatments under various protocols were analyzed for the correlation and agreement of the results. The results indicated that the two assays correlate well in the overlapping linear ranges of the assays and show good agreement. From the results obtained, we have derived a mathematical formula to compare the viral load results between the two assays, which is given as log(10) Abbott assay measure = 0.032 + 1.01 log(10) Bayer assay measure. Although it is preferable to use the same quantitation assay throughout the course of a patient's treatment, valid comparisons of the HCV RNA levels may be made between the results obtained by either of these assays in the overlapping linear range (615 to 7,700,000 IU/ml).
Figures
References
-
- Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, R. E. Sampliner, et al. 1992. The natural history of community-acquired hepatitis C in the United States. N. Engl. J. Med. 327:1899-1905. - PubMed
-
- Benhamou, Y., M. Bochet, V. Di Martino, F. Charlotte, F. Azria, A. Coutellier, M. Vidaud, F. Bricaire, P. Opolon, C. Katlama, T. Poynard, et al. 1999. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology 30:1054-1058. - PubMed
-
- Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. - PubMed
-
- Chung, R. T., J. Andersen, P. Volberding, G. K. Robbins, T. Liu, K. E. Sherman, M. G. Peters, M. J. Koziel, A. K. Bhan, B. Alston, D. Colquhoun, T. Nevin, G. Harb, and C. van der Horst. 2004. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N. Engl. J. Med. 351:451-459. - PMC - PubMed
-
- Elbeik, T., N. Markowitz, P. Nassos, U. Kumar, S. Beringer, B. Haller, and V. Ng. 2004. Simultaneous runs of the Bayer VERSANT HIV-1 version 3.0 and HCV bDNA version 3.0 quantitative assays on the system 340 platform provide reliable quantitation and improved work flow. J. Clin. Microbiol. 42:3120-3127. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

