Comparison of four methods using throat swabs to confirm rubella virus infection
- PMID: 17596370
- PMCID: PMC2045274
- DOI: 10.1128/JCM.00289-07
Comparison of four methods using throat swabs to confirm rubella virus infection
Abstract
Laboratory tests are essential for confirming sporadic cases and outbreaks of rubella. Detection of rubella virus is often necessary to confirm rubella cases and to identify specimens to be used to characterize wild-type rubella viruses. The sensitivities of four methods for detecting rubella virus infection using throat swabs, which had been collected in Henan and Anhui provinces in China, were evaluated. The methods used were reverse transcription (RT)-PCR followed by Southern hybridization using RNA extracted directly from clinical specimens, virus growth in tissue culture followed by virus detection by RT-PCR, low-background immunofluorescence in infected tissue culture cells using monoclonal antibodies to the structural proteins of rubella virus, and a replicon-based method of detecting infectious virus. Among these four methods, direct RT-PCR followed by hybridization was the most sensitive method; the replicon-based method was the least difficult to perform.
Figures
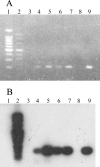

References
-
- Bao, J. S. 2003. Strategy and comparison of incidence of rubella in Luliang County in 2000-2001 disease. Chin. J. Public Health Manage. 19:137-138.
-
- Bellini, W. J., and J. P. Icenogle. 2007. Measles and rubella viruses, p. 1378-1391. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
-
- Bottiger, M., and M. Forsgren. 1997. Twenty years' experience of rubella vaccination in Sweden: 10 years of selective vaccination (of 12-year-old girls and of women postpartum) and 13 years of a general two-dose vaccination. Vaccine 15:1538-1544. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

