Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies
- PMID: 17596426
- PMCID: PMC2044485
- DOI: 10.1128/CVI.00033-07
Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies
Abstract
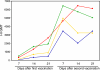
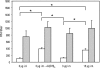
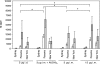
The threat of a new influenza pandemic has led to renewed interest in dose-sparing vaccination strategies such as intradermal immunization and the use of adjuvanted vaccines. In this study we compared the quality and kinetics of the serum antibody response elicited in mice after one or two immunizations with a split influenza A (H3N2) virus, using three different low-dose vaccination strategies. The mice were divided into four groups, receiving either a low-dose vaccine (3 microg hemagglutinin [HA]) intradermally or intramuscularly with or without aluminum adjuvant or the normal human vaccine dose (15 microg HA) intramuscularly. Sera were collected weekly after vaccination and tested in the hemagglutination inhibition, virus neutralization, and enzyme-linked immunosorbent assays. The antibody responses induced after intradermal or intramuscular low-dose vaccinations were similar and lower than those observed after the human vaccine dose. However, low-dose adjuvanted vaccine elicited a serum antibody response comparable to that elicited by the human dose, although the second immunization did not result in any increase in cross-reactive hemagglutination inhibition antibodies, and the peak serum antibody response was observed 1 week later than in the other vaccination groups. Our murine data suggest that the low-dose intradermal route does not show any obvious advantage over the low-dose intramuscular route in inducing a serum antibody response and that none of the low-dose vaccination strategies is as effective as intramuscular vaccination with the normal human dose. However, the low-dose aluminum-adjuvanted vaccine could present a feasible alternative in case of limited vaccine supply.
Figures
References
-
- Auewarakul, P., U. Kositanont, P. Sornsathapornkul, P. Tothong, R. Kanyok, and P. Thongcharoen. 2007. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 25:659-663. - PubMed
-
- Belshe, R. B., F. K. Newman, J. Cannon, C. Duane, J. Treanor, C. Van Hoecke, B. J. Howe, and G. Dubin. 2004. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 351:2286-2294. - PubMed
-
- Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657-1664. - PubMed
-
- Brewer, J. M. 2006. (How) do aluminium adjuvants work? Immunol. Lett. 102:10-15. - PubMed
-
- Cox, R. J., K. A. Brokstad, M. A. Zuckerman, J. M. Wood, L. R. Haaheim, and J. S. Oxford. 1994. An early humoral immune response in peripheral blood following parenteral inactivated influenza vaccination. Vaccine 12:993-999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

