Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons
- PMID: 17596443
- PMCID: PMC6672219
- DOI: 10.1523/JNEUROSCI.0514-07.2007
Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons
Abstract
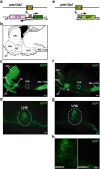
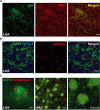
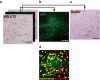
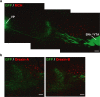
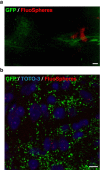
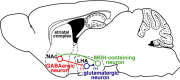
Neuronal circuits including medium spiny neurons (MSNs) in the nucleus accumbens (NAc) and melanin-concentrating hormone (MCH)-containing neurons in the lateral hypothalamic area (LHA) are hypothesized to play an important role in hedonic feeding. A reciprocal connection between NAc MSNs and MCH-containing neurons is proposed to form a neuronal circuit that is involved in hedonic feeding. Although NAc MSNs have been shown to receive projection from MCH-containing neurons, it is not known whether MCH-containing neurons in the LHA also receive direct inputs from NAc MSNs. Here, we developed a genetic approach that allows us to visualize almost all striatal MSNs including NAc MSNs. We demonstrate that striatal MSNs terminate in a distinct region within the anterior LHA, and that the terminal area of striatal MSNs in this region contains glutamatergic neurons and is distinctly separate from orexin/hypocretin- or MCH-containing neurons. These observations suggest that NAc MSNs do not directly innervate MCH-containing neurons, but may indirectly signal MCH-containing neurons via glutamatergic neurons in the anterior LHA.
Figures
Similar articles
-
Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus.J Neurosci. 2008 Sep 10;28(37):9101-10. doi: 10.1523/JNEUROSCI.1766-08.2008. J Neurosci. 2008. PMID: 18784290 Free PMC article.
-
Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons.Cell Mol Neurobiol. 2005 Dec;25(8):1209-23. doi: 10.1007/s10571-005-8184-8. Cell Mol Neurobiol. 2005. PMID: 16388333 Free PMC article.
-
Neurochemical Heterogeneity Among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified Through Single-Cell Gene Expression Analysis.eNeuro. 2017 Sep 22;4(5):ENEURO.0013-17.2017. doi: 10.1523/ENEURO.0013-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 28966976 Free PMC article.
-
Melanin-concentrating hormone neuron system: the Wide Web that controls the feeding.Anat Sci Int. 2002 Sep;77(3):149-60. doi: 10.1046/j.0022-7722.2002.00027.x. Anat Sci Int. 2002. PMID: 12422407 Review.
-
The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells.Physiol Behav. 2021 Feb 1;229:113234. doi: 10.1016/j.physbeh.2020.113234. Epub 2020 Oct 29. Physiol Behav. 2021. PMID: 33130035 Free PMC article. Review.
Cited by
-
Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors.Nat Commun. 2018 Apr 20;9(1):1576. doi: 10.1038/s41467-018-03889-3. Nat Commun. 2018. PMID: 29679009 Free PMC article.
-
The Melanin-Concentrating Hormone as an Integrative Peptide Driving Motivated Behaviors.Front Syst Neurosci. 2017 May 29;11:32. doi: 10.3389/fnsys.2017.00032. eCollection 2017. Front Syst Neurosci. 2017. PMID: 28611599 Free PMC article. Review.
-
The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics.Physiol Behav. 2011 Jul 25;104(1):29-39. doi: 10.1016/j.physbeh.2011.04.051. Epub 2011 May 1. Physiol Behav. 2011. PMID: 21549732 Free PMC article. Review.
-
Identification of optogenetically activated striatal medium spiny neurons by Npas4 expression.PLoS One. 2012;7(12):e52783. doi: 10.1371/journal.pone.0052783. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300775 Free PMC article.
-
GABAergic neurons in the nucleus accumbens regulate hedonic food intake via orexin-A expression in the lateral hypothalamus.Iran J Basic Med Sci. 2021 Sep;24(9):1272-1278. doi: 10.22038/ijbms.2021.58140.12923. Iran J Basic Med Sci. 2021. PMID: 35083015 Free PMC article.
References
-
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. - PubMed
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. - PubMed
-
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
