Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice
- PMID: 17596451
- PMCID: PMC6672226
- DOI: 10.1523/JNEUROSCI.1235-07.2007
Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice
Abstract
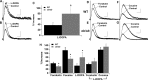
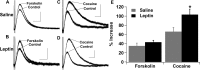
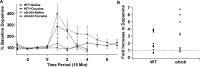
An increasing number of studies indicate that leptin can regulate the activity of the mesolimbic dopamine system. The objective of this study was to examine the regulation of the activity of dopamine neurons by leptin. This was accomplished by examining the dopamine D2 receptor-mediated synaptic current that resulted from somatodendritic release of dopamine in brain slices taken from mice that lacked leptin (Lep(ob/ob) mice). Under control conditions, the amplitude and kinetics of the IPSC in wild-type and Lep(ob/ob) mice were not different. However, in the presence of forskolin or cocaine, the facilitation of the dopamine IPSC was significantly reduced in Lep(ob/ob) mice. The application of L-3,4-dihydroxyphenylalanine (L-DOPA) increased the IPSC in Lep(ob/ob) mice significantly more than in wild-type animals and fully restored the responses to both forskolin and cocaine. Treatment of Lep(ob/ob) mice with leptin in vivo fully restored the cocaine-induced increase in the IPSC to wild-type levels. These results suggest that there is a decrease in the content of somatodendritic vesicular dopamine in the Lep(ob/ob) mice. The release of dopamine from terminals may be less affected in the Lep(ob/ob) mice, because the cocaine-induced rise in dopamine in the ventral striatum was not statistically different between wild-type and Lep(ob/ob) mice. In addition, the relative increase in cocaine-induced locomotion was similar for wild-type and Lep(ob/ob) mice. These results indicate that, although basal release is not altered, the amount of dopamine that can be released is reduced in Lep(ob/ob) mice.
Figures


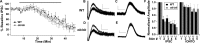
References
-
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. - PubMed
-
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. - PubMed
-
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. - PubMed
-
- Calcagnetti DJ, Flynn JJ, Margules DL. Opioid-induced linear running in obese (ob/ob) and lean mice. Pharmacol Biochem Behav. 1987;26:743–747. - PubMed
-
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
