State-dependent modulation of amygdala inputs by dopamine-induced enhancement of sodium currents in layer V entorhinal cortex
- PMID: 17596455
- PMCID: PMC6672235
- DOI: 10.1523/JNEUROSCI.1744-07.2007
State-dependent modulation of amygdala inputs by dopamine-induced enhancement of sodium currents in layer V entorhinal cortex
Abstract
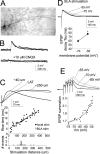
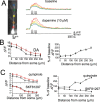
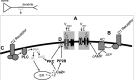
Interaction between the entorhinal cortex (EC) and basolateral amygdala (BLA) may be a fundamental component in the consolidation of many forms of affective memory, such as inhibitory avoidance. Dopamine (DA) in the EC is necessary for, and may facilitate, this form of learning. This effect of DA on affective behaviors may be accomplished in part through modulation of amygdala inputs. Although it is known that DA can modulate neuronal activity in the EC, it is not known whether DA modulates inputs from the BLA. In this study, we used in vitro patch-clamp recordings and Ca2+ imaging of layer V neurons in the rat lateral EC to determine whether DA modulates the integration of inputs from the BLA and the mechanism for this modulation. We found that DA exerted actions that depended on the neuronal state. Near resting membrane potentials, DA suppressed integration of inputs, whereas at depolarized potentials, DA enhanced integration. DA enhanced the integration by a D2-mediated enhancement of Na+ currents, via phospholipase C. These experiments demonstrate that DA can exert actions in the EC that depend on the membrane voltage. This effect of DA may affect a wide range of inputs. Functionally, by enhancement of amygdala inputs that arrive in concert with other inputs, or during depolarized states, DA can facilitate the impact of affect on memory in a subset of conditions.
Figures








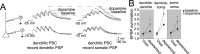
Similar articles
-
Dopaminergic regulation of neuronal excitability through modulation of Ih in layer V entorhinal cortex.J Neurosci. 2006 Mar 22;26(12):3229-44. doi: 10.1523/JNEUROSCI.4333-05.2006. J Neurosci. 2006. PMID: 16554474 Free PMC article.
-
Dopamine modulates excitability of basolateral amygdala neurons in vitro.J Neurophysiol. 2005 Mar;93(3):1598-610. doi: 10.1152/jn.00843.2004. Epub 2004 Nov 10. J Neurophysiol. 2005. PMID: 15537813
-
Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission.Eur J Neurosci. 2012 Jan;35(2):279-90. doi: 10.1111/j.1460-9568.2011.07943.x. Epub 2012 Jan 12. Eur J Neurosci. 2012. PMID: 22236063
-
Regulation of conditioned responses of basolateral amygdala neurons.Physiol Behav. 2002 Dec;77(4-5):489-93. doi: 10.1016/s0031-9384(02)00909-5. Physiol Behav. 2002. PMID: 12526988 Review.
-
Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior.Neurosci Biobehav Rev. 2003 Oct;27(6):543-54. doi: 10.1016/j.neubiorev.2003.09.002. Neurosci Biobehav Rev. 2003. PMID: 14599435 Review.
Cited by
-
Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo.Neuropsychopharmacology. 2013 Aug;38(9):1748-62. doi: 10.1038/npp.2013.74. Epub 2013 Mar 27. Neuropsychopharmacology. 2013. PMID: 23535779 Free PMC article.
-
Functional maps within a single neuron.J Neurophysiol. 2012 Nov;108(9):2343-51. doi: 10.1152/jn.00530.2012. Epub 2012 Aug 29. J Neurophysiol. 2012. PMID: 22933729 Free PMC article. Review.
-
Distance- and activity-dependent modulation of spike back-propagation in layer V pyramidal neurons of the medial entorhinal cortex.J Neurophysiol. 2011 Mar;105(3):1372-9. doi: 10.1152/jn.00014.2010. Epub 2011 Jan 5. J Neurophysiol. 2011. PMID: 21209358 Free PMC article.
-
The enigmatic HCN channels: A cellular neurophysiology perspective.Proteins. 2025 Jan;93(1):72-92. doi: 10.1002/prot.26643. Epub 2023 Nov 19. Proteins. 2025. PMID: 37982354 Free PMC article. Review.
-
Dopamine and full-field illumination activate D1 and D2-D5-type receptors in adult rat retinal ganglion cells.J Comp Neurol. 2012 Dec 1;520(17):4032-49. doi: 10.1002/cne.23159. J Comp Neurol. 2012. PMID: 22678972 Free PMC article.
References
-
- Agrawal N, Hamam BN, Magistretti J, Alonso A, Ragsdale DS. Persistent sodium channel activity mediates subthreshold membrane potential oscillations and low-threshold spikes in rat entorhinal cortex layer V neurons. Neuroscience. 2001;102:53–64. - PubMed
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. - PubMed
-
- Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry. 2000;47:361–370. - PubMed
-
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. - PubMed
-
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
