Dual regulation of TRPV1 by phosphoinositides
- PMID: 17596456
- PMCID: PMC6672228
- DOI: 10.1523/JNEUROSCI.1866-07.2007
Dual regulation of TRPV1 by phosphoinositides
Abstract
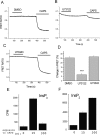
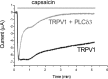
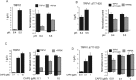
The membrane phospholipid phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2 or PIP2] regulates many ion channels. There are conflicting reports on the effect of PtdIns(4,5)P2 on transient receptor potential vanilloid 1 (TRPV1) channels. We show that in excised patches PtdIns(4,5)P2 and other phosphoinositides activate and the PIP2 scavenger poly-Lys inhibits TRPV1. TRPV1 currents undergo desensitization on exposure to high concentrations of capsaicin in the presence of extracellular Ca2+. We show that in the presence of extracellular Ca2+, capsaicin activates phospholipase C (PLC) in TRPV1-expressing cells, inducing depletion of both PtdIns(4,5)P2 and its precursor PtdIns(4)P (PIP). The PLC inhibitor U73122 and dialysis of PtdIns(4,5)P2 or PtdIns(4)P through the patch pipette inhibited desensitization of TRPV1, indicating that Ca2+-induced activation of PLC contributes to desensitization of TRPV1 by depletion of PtdIns(4,5)P2 and PtdIns(4)P. Selective conversion of PtdIns(4,5)P2 to PtdIns(4)P by a rapamycin-inducible PIP2 5-phosphatase did not inhibit TRPV1 at high capsaicin concentrations, suggesting a significant role for PtdIns(4)P in maintaining channel activity. Currents induced by low concentrations of capsaicin and moderate heat, however, were potentiated by conversion of PtdIns(4,5)P2 to PtdIns(4)P. Increasing PtdIns(4,5)P2 levels by coexpressing phosphatidylinositol-4-phosphate 5-kinase inhibited TRPV1 at low but not at saturating capsaicin concentrations. These data show that at low capsaicin concentrations and other moderate stimuli, PtdIns(4,5)P2 partially inhibits TRPV1 in a cellular context, but this effect is likely to be indirect, because it is not detectable in excised patches. We conclude that phosphoinositides have both inhibitory and activating effects on TRPV1, resulting in complex and distinct regulation at various stimulation levels.
Figures






Similar articles
-
Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides.Sci Signal. 2015 Feb 10;8(363):ra15. doi: 10.1126/scisignal.2005667. Sci Signal. 2015. PMID: 25670203 Free PMC article.
-
Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons.J Neurosci. 2013 Jul 10;33(28):11451-63. doi: 10.1523/JNEUROSCI.5637-12.2013. J Neurosci. 2013. PMID: 23843517 Free PMC article.
-
Dual regulation of TRPV1 channels by phosphatidylinositol via functionally distinct binding sites.J Biol Chem. 2021 Jan-Jun;296:100573. doi: 10.1016/j.jbc.2021.100573. Epub 2021 Mar 23. J Biol Chem. 2021. PMID: 33766560 Free PMC article.
-
Phospholipase C mediated modulation of TRPV1 channels.Mol Neurobiol. 2008 Apr-Jun;37(2-3):153-63. doi: 10.1007/s12035-008-8027-y. Epub 2008 Jun 5. Mol Neurobiol. 2008. PMID: 18528787 Free PMC article. Review.
-
Phosphoinositide regulation of TRPV1 revisited.Pflugers Arch. 2015 Sep;467(9):1851-69. doi: 10.1007/s00424-015-1695-3. Epub 2015 Mar 11. Pflugers Arch. 2015. PMID: 25754030 Free PMC article. Review.
Cited by
-
TRPV1: A Target for Rational Drug Design.Pharmaceuticals (Basel). 2016 Aug 23;9(3):52. doi: 10.3390/ph9030052. Pharmaceuticals (Basel). 2016. PMID: 27563913 Free PMC article. Review.
-
Construction of a global pain systems network highlights phospholipid signaling as a regulator of heat nociception.PLoS Genet. 2012;8(12):e1003071. doi: 10.1371/journal.pgen.1003071. Epub 2012 Dec 6. PLoS Genet. 2012. PMID: 23236288 Free PMC article.
-
The Membrane Proximal Domain of TRPV1 and TRPV2 Channels Mediates Protein⁻Protein Interactions and Lipid Binding In Vitro.Int J Mol Sci. 2019 Feb 5;20(3):682. doi: 10.3390/ijms20030682. Int J Mol Sci. 2019. PMID: 30764505 Free PMC article.
-
Localization of the PIP2 sensor of TRPV1 ion channels.J Biol Chem. 2011 Mar 18;286(11):9688-98. doi: 10.1074/jbc.M110.192526. Epub 2011 Jan 11. J Biol Chem. 2011. PMID: 21224382 Free PMC article.
-
Diacylglycerol kinases regulate TRPV1 channel activity.J Biol Chem. 2020 Jun 12;295(24):8174-8185. doi: 10.1074/jbc.RA119.012505. Epub 2020 Apr 28. J Biol Chem. 2020. PMID: 32345612 Free PMC article.
References
-
- Balla T. Pharmacology of phosphoinositides, regulators of multiple cellular functions. Curr Pharm Des. 2001;7:475–507. - PubMed
-
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998:1141–1144. - PubMed
-
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. - PubMed
-
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
