An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system
- PMID: 17596510
- PMCID: PMC1951749
- DOI: 10.1091/mbc.e07-03-0193
An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system
Abstract
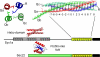
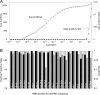
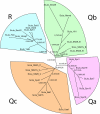
Proteins of the SNARE (soluble N-ethylmalemide-sensitive factor attachment protein receptor) family are essential for the fusion of transport vesicles with an acceptor membrane. Despite considerable sequence divergence, their mechanism of action is conserved: heterologous sets assemble into membrane-bridging SNARE complexes, in effect driving membrane fusion. Within the cell, distinct functional SNARE units are involved in different trafficking steps. These functional units are conserved across species and probably reflect the conservation of the particular transport step. Here, we have systematically analyzed SNARE sequences from 145 different species and have established a highly accurate classification for all SNARE proteins. Principally, all SNAREs split into four basic types, reflecting their position in the four-helix bundle complex. Among these four basic types, we established 20 SNARE subclasses that probably represent the original repertoire of a eukaryotic cenancestor. This repertoire has been modulated independently in different lines of organisms. Our data are in line with the notion that the ur-eukaryotic cell was already equipped with the various compartments found in contemporary cells. Possibly, the development of these compartments is closely intertwined with episodes of duplication and divergence of a prototypic SNARE unit.
Figures
References
-
- Antonin W., Dulubova I., Arac D., Pabst S., Plitzner J., Rizo J., Jahn R. The N-terminal domains of syntaxin 7 and vti1b form three-helix bundles that differ in their ability to regulate SNARE complex assembly. J. Biol. Chem. 2002a;277:36449–36456. - PubMed
-
- Antonin W., Fasshauer D., Becker S., Jahn R., Schneider T. R. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat. Struct. Biol. 2002b;9:107–111. - PubMed
-
- Aury J. M., et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature. 2006;444:171–178. - PubMed
-
- Ayong L., Pagnotti G., Tobon A. B., Chakrabarti D. Identification of Plasmodium falciparum family of SNAREs. Mol. Biochem. Parasitol. 2007;152:113–122. - PubMed
-
- Banfield D. K. SNARE complexes—is there sufficient complexity for vesicle targeting specificity? Trends Biochem. Sci. 2001;26:67–68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

