The trans-Golgi network accessory protein p56 promotes long-range movement of GGA/clathrin-containing transport carriers and lysosomal enzyme sorting
- PMID: 17596511
- PMCID: PMC1951763
- DOI: 10.1091/mbc.e07-02-0190
The trans-Golgi network accessory protein p56 promotes long-range movement of GGA/clathrin-containing transport carriers and lysosomal enzyme sorting
Abstract
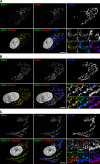
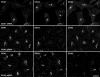
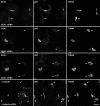
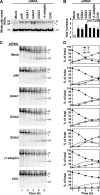
The sorting of acid hydrolase precursors at the trans-Golgi network (TGN) is mediated by binding to mannose 6-phosphate receptors (MPRs) and subsequent capture of the hydrolase-MPR complexes into clathrin-coated vesicles or transport carriers (TCs) destined for delivery to endosomes. This capture depends on the function of three monomeric clathrin adaptors named GGAs. The GGAs comprise a C-terminal "ear" domain that binds a specific set of accessory proteins. Herein we show that one of these accessory proteins, p56, colocalizes and physically interacts with the three GGAs at the TGN. Moreover, overexpression of the GGAs enhances the association of p56 with the TGN, and RNA interference (RNAi)-mediated depletion of the GGAs decreases the TGN association and total levels of p56. RNAi-mediated depletion of p56 or the GGAs causes various degrees of missorting of the precursor of the acid hydrolase, cathepsin D. In the case of p56 depletion, this missorting correlates with decreased mobility of GGA-containing TCs. Transfection with an RNAi-resistant p56 construct, but not with a p56 construct lacking the GGA-ear-interacting motif, restores the mobility of the TCs. We conclude that p56 tightly cooperates with the GGAs in the sorting of cathepsin D to lysosomes, probably by enabling the movement of GGA-containing TCs.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

