LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction
- PMID: 17596512
- PMCID: PMC1951746
- DOI: 10.1091/mbc.e07-02-0124
LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction
Abstract
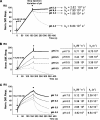
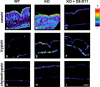
LEKTI is a 15-domain serine proteinase inhibitor whose defective expression underlies the severe autosomal recessive ichthyosiform skin disease, Netherton syndrome. Here, we show that LEKTI is produced as a precursor rapidly cleaved by furin, generating a variety of single or multidomain LEKTI fragments secreted in cultured keratinocytes and in the epidermis. The identity of these biological fragments (D1, D5, D6, D8-D11, and D9-D15) was inferred from biochemical analysis, using a panel of LEKTI antibodies. The functional inhibitory capacity of each fragment was tested on a panel of serine proteases. All LEKTI fragments, except D1, showed specific and differential inhibition of human kallikreins 5, 7, and 14. The strongest inhibition was observed with D8-D11, toward KLK5. Kinetics analysis revealed that this interaction is rapid and irreversible, reflecting an extremely tight binding complex. We demonstrated that pH variations govern this interaction, leading to the release of active KLK5 from the complex at acidic pH. These results identify KLK5, a key actor of the desquamation process, as the major target of LEKTI. They disclose a new mechanism of skin homeostasis by which the epidermal pH gradient allows precisely regulated KLK5 activity and corneodesmosomal cleavage in the most superficial layers of the stratum corneum.
Figures





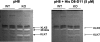

References
-
- Ahmed A., Kandola P., Ziada G., Parenteau N. Purification and partial amino acid sequence of proteins from human epidermal keratinocyte conditioned medium. J. Protein Chem. 2001;20:273–278. - PubMed
-
- Bitoun E., et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Hum. Mol. Genet. 2003;12:2417–2430. - PubMed
-
- Brattsand M., Stefansson K., Lundh C., Haasum Y., Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J. Invest. Dermatol. 2005;124:198–203. - PubMed
-
- Caubet C., Jonca N., Brattsand M., Guerrin M., Bernard D., Schmidt R., Egelrud T., Simon M., Serre G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 2004;122:1235–1244. - PubMed
-
- Chavanas S., et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause netherton syndrome. Nat. Genet. 2000;25:141–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

