A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway
- PMID: 17596516
- PMCID: PMC1951772
- DOI: 10.1091/mbc.e07-01-0053
A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway
Abstract
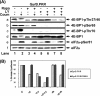
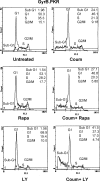
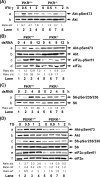
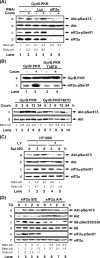
Phosphoinositide-3 kinase (PI3K) plays an important role in signal transduction in response to a wide range of cellular stimuli involved in cellular processes that promote cell proliferation and survival. Phosphorylation of the alpha subunit of the eukaryotic translation initiation factor eIF2 at Ser51 takes place in response to various types of environmental stress and is essential for regulation of translation initiation. Herein, we show that a conditionally active form of the eIF2alpha kinase PKR acts upstream of PI3K and turns on the Akt/PKB-FRAP/mTOR pathway leading to S6 and 4E-BP1 phosphorylation. Also, induction of PI3K signaling antagonizes the apoptotic and protein synthesis inhibitory effects of the conditionally active PKR. Furthermore, induction of the PI3K pathway is impaired in PKR(-/-) or PERK(-/-) mouse embryonic fibroblasts (MEFs) in response to various stimuli that activate each eIF2alpha kinase. Mechanistically, PI3K signaling activation is indirect and requires the inhibition of protein synthesis by eIF2alpha phosphorylation as demonstrated by the inactivation of endogenous eIF2alpha by small interfering RNA or utilization of MEFs bearing the eIF2alpha Ser51Ala mutation. Our data reveal a novel property of eIF2alpha kinases as activators of PI3K signaling and cell survival.
Figures



Similar articles
-
Akt determines cell fate through inhibition of the PERK-eIF2α phosphorylation pathway.Sci Signal. 2011 Sep 27;4(192):ra62. doi: 10.1126/scisignal.2001630. Sci Signal. 2011. PMID: 21954288 Free PMC article.
-
Control of oncogenesis by eIF2α phosphorylation: implications in PTEN and PI3K-Akt signaling and tumor treatment.Future Oncol. 2013 Jul;9(7):1005-15. doi: 10.2217/fon.13.49. Future Oncol. 2013. PMID: 23837763 Review.
-
Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death.Mol Cell Biol. 2004 Apr;24(8):3415-29. doi: 10.1128/MCB.24.8.3415-3429.2004. Mol Cell Biol. 2004. PMID: 15060162 Free PMC article.
-
Involvement of ERKs, RSK2 and PKR in UVA-induced signal transduction toward phosphorylation of eIF2alpha (Ser(51)).Carcinogenesis. 2007 Jul;28(7):1543-51. doi: 10.1093/carcin/bgm070. Epub 2007 Apr 2. Carcinogenesis. 2007. PMID: 17404396
-
The eIF2α kinases: their structures and functions.Cell Mol Life Sci. 2013 Oct;70(19):3493-511. doi: 10.1007/s00018-012-1252-6. Epub 2013 Jan 26. Cell Mol Life Sci. 2013. PMID: 23354059 Free PMC article. Review.
Cited by
-
Role of the ISR-ATF4 pathway and its cross talk with Nrf2 in mitochondrial quality control.J Clin Biochem Nutr. 2019 Jan;64(1):1-12. doi: 10.3164/jcbn.18-37. Epub 2018 Sep 15. J Clin Biochem Nutr. 2019. PMID: 30705506 Free PMC article. Review.
-
Stress and IGF-I differentially control cell fate through mammalian target of rapamycin (mTOR) and retinoblastoma protein (pRB).J Biol Chem. 2008 Oct 17;283(42):28265-73. doi: 10.1074/jbc.M805724200. Epub 2008 Aug 11. J Biol Chem. 2008. PMID: 18697743 Free PMC article.
-
Hydrogen sulfide ameliorates abdominal aorta coarctation-induced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2α phosphorylation and activating PI3K/AKT1 pathway.Korean J Physiol Pharmacol. 2023 Jul 1;27(4):345-356. doi: 10.4196/kjpp.2023.27.4.345. Korean J Physiol Pharmacol. 2023. PMID: 37386832 Free PMC article.
-
The integrated stress response.EMBO Rep. 2016 Oct;17(10):1374-1395. doi: 10.15252/embr.201642195. Epub 2016 Sep 14. EMBO Rep. 2016. PMID: 27629041 Free PMC article. Review.
-
PKR regulates B56(alpha)-mediated BCL2 phosphatase activity in acute lymphoblastic leukemia-derived REH cells.J Biol Chem. 2008 Dec 19;283(51):35474-85. doi: 10.1074/jbc.M800951200. Epub 2008 Oct 28. J Biol Chem. 2008. PMID: 18957415 Free PMC article.
References
-
- Abraham N., et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 1999;274:5953–5962. - PubMed
-
- Abraham R. T. PI 3-kinase related kinases: ‘big’ players in stress-induced signaling pathways. DNA Repair (Amst.) 2004;3:883–887. - PubMed
-
- Baltzis D., Li S., Koromilas A. E. Functional characterization of pkr gene products expressed in cells from mice with a targeted deletion of the N terminus or C terminus domain of PKR. J. Biol. Chem. 2002;277:38364–38372. - PubMed
-
- Boyce M., et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

