Hypoxia/reoxygenation of isolated rat heart mitochondria causes cytochrome c release and oxidative stress; evidence for involvement of mitochondrial nitric oxide synthase
- PMID: 17597148
- PMCID: PMC2045686
- DOI: 10.1016/j.yjmcc.2007.05.019
Hypoxia/reoxygenation of isolated rat heart mitochondria causes cytochrome c release and oxidative stress; evidence for involvement of mitochondrial nitric oxide synthase
Abstract
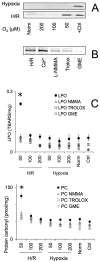
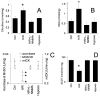
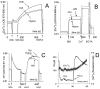
The objective of the present study was to delineate the molecular mechanisms for mitochondrial contribution to oxidative stress induced by hypoxia and reoxygenation in the heart. The present study introduces a novel model allowing real-time study of mitochondria under hypoxia and reoxygenation, and describes the significance of intramitochondrial calcium homeostasis and mitochondrial nitric oxide synthase (mtNOS) for oxidative stress. The present study shows that incubating isolated rat heart mitochondria under hypoxia followed by reoxygenation, but not hypoxia per se, causes cytochrome c release from the mitochondria, oxidative modification of mitochondrial lipids and proteins, and inactivation of mitochondrial enzymes susceptible to inactivation by peroxynitrite. These alterations were prevented when mtNOS was inhibited or mitochondria were supplemented with antioxidant peroxynitrite scavengers. The present study shows mitochondria independent of other cellular components respond to hypoxia/reoxygenation by elevating intramitochondrial ionized calcium and stimulating mtNOS. The present study proposes a crucial role for heart mitochondrial calcium homeostasis and mtNOS in oxidative stress induced by hypoxia/reoxygenation.
Figures



Comment in
-
Heart mtNOS, a key mediator of oxidative injury in ischemia/reperfusion.J Mol Cell Cardiol. 2007 Oct;43(4):409-10. doi: 10.1016/j.yjmcc.2007.07.053. Epub 2007 Jul 24. J Mol Cell Cardiol. 2007. PMID: 17764688 No abstract available.
References
-
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–41. - PubMed
-
- Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–89. - PubMed
-
- Pi Y, Goldenthal MJ, Marin-Garcia J. Mitochondrial involvement in IGF-1 induced protection of cardiomyocytes against hypoxia/reoxygenation injury. Mol Cell Biochem. 2007 in press. - PubMed
-
- Gottlieb RA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann N Y Acad Sci. 1999;874:412–26. - PubMed
-
- Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26:190–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

