Protein-protein Förster resonance energy transfer analysis of nucleosome core particles containing H2A and H2A.Z
- PMID: 17597150
- PMCID: PMC2031861
- DOI: 10.1016/j.jmb.2007.05.075
Protein-protein Förster resonance energy transfer analysis of nucleosome core particles containing H2A and H2A.Z
Abstract
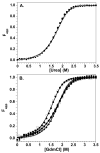
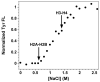
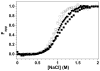
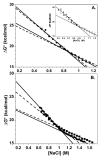
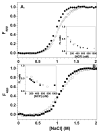
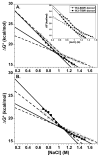
A protein-protein Förster resonance energy transfer (FRET) system, employing probes at multiple positions, was designed to specifically monitor the dissociation of the H2A-H2B dimer from the nucleosome core particle (NCP). Tryptophan donors and Cys-AEDANS acceptors were chosen because, compared to previous NCP FRET fluorophores, they: (1) are smaller and less hydrophobic, which should minimize perturbations of histone and NCP structure; and (2) have an R0 of 20 A, which is much less than the dimensions of the NCP (approximately 50 A width and approximately 100 A diameter). Equilibrium protein unfolding titrations indicate that the donor and acceptor moieties have minimal effects on the stability of the H2A-H2B dimer and (H3-H4)2 tetramer. NCPs containing the various FRET pairs were reconstituted with the 601 DNA positioning element. Equilibrium NaCl-induced dissociation of the modified NCPs showed that the 601 sequence stabilized the NCP to dimer dissociation relative to weaker positioning sequences. This finding implies a significant role for the H2A-H2B dimers in determining the DNA sequence dependence of NCP stability. The free energy of dissociation determined from reversible and well-defined sigmoidal transitions revealed two distinct phases reflecting the dissociation of individual H2A-H2B dimers, confirming cooperativity as suggested previously; these data allow quantitative description of the cooperativity. The FRET system was then used to study the effects of the histone variant H2A.Z on NCP stability; previous studies have reported both destabilizing and stabilizing effects. H2A.Z FRET NCP dissociation transitions suggest a slight increase in stability but a significant increase in cooperativity of the dimer dissociations. Thus, the utility of this protein-protein FRET system to monitor the effects of histone variants on NCP dynamics has been demonstrated, and the system appears equally well-suited for dissection of the kinetic processes of dimer association and dissociation from the NCP.
Figures


References
-
- Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Ann Rev Biochem. 1998;67:545–79. - PubMed
-
- Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell. 2004;116:259–72. - PubMed
-
- Luger K. Dynamic nucleosomes. Chromosome Res. 2006;14:5–16. - PubMed
-
- Wolffe AP. Chromatin remodeling: why it is important in cancer. Oncogene. 2001;20:2988–90. - PubMed
-
- Huang C, Sloan EA, Boerkoel CF. Chromatin remodeling and human disease. Curr Opin Genet Dev. 2003;13:246–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

