Characterization of the first angiotensin-converting like enzyme in bacteria: Ancestor ACE is already active
- PMID: 17597310
- PMCID: PMC7127174
- DOI: 10.1016/j.gene.2007.05.010
Characterization of the first angiotensin-converting like enzyme in bacteria: Ancestor ACE is already active
Abstract
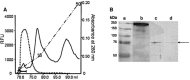
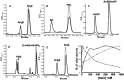
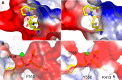
Angiotensin-converting enzyme (ACE) is a metallopeptidase that converts angiotensin I into angiotensin II. ACE is crucial in the control of cardiovascular and renal homeostasis and fertility in mammals. In vertebrates, both transmembrane and soluble ACE, containing one or two active sites, have been characterized. So far, only soluble, single domain ACEs from invertebrates have been cloned, and these have been implicated in reproduction in insects. Furthermore, an ACE-related carboxypeptidase was recently characterized in Leishmania, a unicellular eukaryote, suggesting the existence of ACE in more distant organisms. Interestingly, in silico databank analysis revealed that bacterial DNA sequences could encode putative ACE-like proteins, strikingly similar to vertebrates' enzymes. To gain more insight into the bacterial enzymes, we cloned the putative ACE from the phytopathogenic bacterium, Xanthomonas axonopodis pv. citri, named XcACE. The 2 kb open reading frame encodes a 672-amino-acid soluble protein containing a single active site. In vitro expression and biochemical characterization revealed that XcACE is a functional 72 kDa dipeptidyl-carboxypeptidase. As in mammals, this metalloprotease hydrolyses angiotensin I into angiotensin II. XcACE is sensitive to ACE inhibitors and chloride ions concentration. Variations in the active site residues, highlighted by structural modelling, can account for the different substrate selectivity and inhibition profile compared to human ACE. XcACE characterization demonstrates that ACE is an ancestral enzyme, provoking questions about its appearance and structure/activity specialisation during the course of evolution.
Figures

Similar articles
-
Characterization of the first non-insect invertebrate functional angiotensin-converting enzyme (ACE): leech TtACE resembles the N-domain of mammalian ACE.Biochem J. 2004 Sep 1;382(Pt 2):565-73. doi: 10.1042/BJ20040522. Biochem J. 2004. PMID: 15175004 Free PMC article.
-
A crucial role in fertility for the oyster angiotensin-converting enzyme orthologue CgACE.PLoS One. 2011;6(12):e27833. doi: 10.1371/journal.pone.0027833. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174750 Free PMC article.
-
[Angiotensin-converting enzyme: a protein conserved during evolution].J Soc Biol. 2009;203(4):281-93. doi: 10.1051/jbio/2009032. Epub 2010 Feb 1. J Soc Biol. 2009. PMID: 20122386 Review. French.
-
Residues affecting the chloride regulation and substrate selectivity of the angiotensin-converting enzymes (ACE and ACE2) identified by site-directed mutagenesis.FEBS J. 2008 Dec;275(23):6033-42. doi: 10.1111/j.1742-4658.2008.06733.x. FEBS J. 2008. PMID: 19021774 Free PMC article.
-
New aspects on angiotensin-converting enzyme: from gene to disease.Clin Chem Lab Med. 2002 Mar;40(3):256-65. doi: 10.1515/CCLM.2002.042. Clin Chem Lab Med. 2002. PMID: 12005216 Review.
Cited by
-
Are antibacterial effects of non-antibiotic drugs random or purposeful because of a common evolutionary origin of bacterial and mammalian targets?Infection. 2021 Aug;49(4):569-589. doi: 10.1007/s15010-020-01547-9. Epub 2020 Dec 15. Infection. 2021. PMID: 33325009 Free PMC article. Review.
-
Altitudinal variation of the gut microbiota in wild house mice.Mol Ecol. 2019 May;28(9):2378-2390. doi: 10.1111/mec.14905. Epub 2018 Nov 15. Mol Ecol. 2019. PMID: 30346069 Free PMC article.
-
An update on angiotensin-converting enzyme 2 structure/functions, polymorphism, and duplicitous nature in the pathophysiology of coronavirus disease 2019: Implications for vascular and coagulation disease associated with severe acute respiratory syndrome coronavirus infection.Front Microbiol. 2022 Nov 28;13:1042200. doi: 10.3389/fmicb.2022.1042200. eCollection 2022. Front Microbiol. 2022. PMID: 36519165 Free PMC article. Review.
-
Regulation of cell volume and water transport--an old fundamental role of the renin angiotensin aldosterone system components at the cellular level.Peptides. 2014 Aug;58:74-7. doi: 10.1016/j.peptides.2014.06.003. Epub 2014 Jun 16. Peptides. 2014. PMID: 24945466 Free PMC article. Review.
-
Genetic changes involving the coral gastrovascular system support the transition between colonies and bailed-out polyps: evidence from a Pocillopora acuta transcriptome.BMC Genomics. 2021 Sep 26;22(1):694. doi: 10.1186/s12864-021-08026-x. BMC Genomics. 2021. PMID: 34563133 Free PMC article.
References
-
- Azizi M., Massien C., Michaud A., Corvol P. In vitro and in vivo inhibition of the 2 active sites of ACE by omapatrilat, a vasopeptidase inhibitor. Hypertension. 2000;35:1226–1231. - PubMed
-
- Brooks D.R., Appleford P.J., Murray L., Isaac R.E. An essential role in moulting and morphogenesis of Caenorhabditis elegans for ACN-1: a novel member of the angiotensin-converting enzyme family that lacks a metallopeptidase active site. J Biol. Chem. 2003;278:52340–52346. - PubMed
-
- Coates D. Functional conservation of the active sites of human and Drosophila angiotensin I-converting enzyme. Biochemistry. 2000;39:8963–8969. - PubMed
-
- Cornell M.J. Cloning and expression of an evolutionary conserved single-domain angiotensin converting enzyme from Drosophila melanogaster. J. Biol. Chem. 1995;270:13613–13619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

