Maternal antibody and viral factors in the pathogenesis of dengue virus in infants
- PMID: 17597456
- PMCID: PMC4333207
- DOI: 10.1086/519170
Maternal antibody and viral factors in the pathogenesis of dengue virus in infants
Abstract
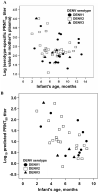
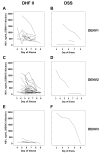
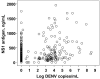
The pathogenesis of dengue in infants is poorly understood. We postulated that dengue severity in infants would be positively associated with markers of viral burden and that maternally derived, neutralizing anti-dengue antibody would have decayed before the age at which infants with dengue presented to the hospital. In 75 Vietnamese infants with primary dengue, we found significant heterogeneity in viremia and NS1 antigenemia at hospital presentation, and these factors were independent of disease grade or continuous measures of disease severity. Neutralizing antibody titers, predicted in each infant at the time of their illness, suggested that the majority of infants (65%) experienced dengue hemorrhagic fever when the maternally derived neutralizing antibody titer had declined to <1 : 20. Collectively, these data have important implications for dengue vaccine research because they suggest that viral burden may not solely explain severe dengue in infants and that neutralizing antibody is a reasonable but not absolute marker of protective immunity in infants.
Figures



References
-
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. - PubMed
-
- Graham RR, Juffrie M, Tan R, et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia. I. Studies in 1995–1996. Am J Trop Med Hyg. 1999;61:412–9. - PubMed
-
- Thein S, Aung MM, Shwe TN, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56:566–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

