Variability in effective radiating area and output power of new ultrasound transducers at 3 MHz
- PMID: 17597939
- PMCID: PMC1896073
Variability in effective radiating area and output power of new ultrasound transducers at 3 MHz
Abstract
Context: Spatial average intensity (SAI) is often used by clinicians to gauge therapeutic ultrasound dosage, yet SAI measures are not directly regulated by US Food and Drug Administration (FDA) standards. Current FDA guidelines permit a possible 50% to 150% minimum to maximum range of SAI values, potentially contributing to variability in clinical outcomes.
Objective: To measure clinical values that describe ultrasound transducers and to determine the degree of intramanufacturer and intermanufacturer variability in effective radiating area, power, and SAI when the transducer is functioning at 3 MHz.
Design: A descriptive and interferential approach was taken to this quasi-experimental design.
Setting: Measurement laboratory.
Patients or other participants: Sixty-six 5-cm(2) ultrasound transducers were purchased from 6 different manufacturers.
Intervention(s): All transducers were calibrated and then assessed using standardized measurement techniques; SAI was normalized to account for variability in effective radiating area, resulting in an nSAI.
Main outcome measure(s): Effective radiating area, power, and nSAI.
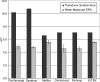
Results: All manufacturers with the exception of Omnisound (P = .534) showed a difference between the reported and measured effective radiating area values (P < .001). All transducers were within FDA guidelines for power (+/-20%). Chattanooga (0.85 +/- 0.05 W/cm(2)) had a lower nSAI (P < .05) than all other manufacturers functioning at 3 MHz. Intramanufacturer variability in SAI ranged from 16% to 35%, and intermanufacturer variability ranged from 22% to 61%.
Conclusions: Clinicians should consider treatment values of each individual transducer, regardless of the manufacturer. In addition, clinicians should scrutinize the power calibration and recalibration record of the transducer and adjust clinical settings as needed for the desired level of heating. Our data may aid in explaining the reported heating differences among transducers from different manufacturers. Stricter FDA standards regarding effective radiating area and total power are needed, and standards regulating SAI should be established.
Figures
Similar articles
-
Variability in effective radiating area at 1 MHz affects ultrasound treatment intensity.Phys Ther. 2008 Jan;88(1):50-7. doi: 10.2522/ptj.20060358. Epub 2007 Oct 16. Phys Ther. 2008. PMID: 17940107
-
Analysis of effective radiating area, power, intensity, and field characteristics of ultrasound transducers.Arch Phys Med Rehabil. 2007 Jan;88(1):124-9. doi: 10.1016/j.apmr.2006.09.016. Arch Phys Med Rehabil. 2007. PMID: 17207688
-
The importance of measurement of effective transducers radiating area in the testing and calibration of "therapeutic" ultrasonic instruments.Health Phys. 1982 Sep;43(3):377-81. doi: 10.1097/00004032-198209000-00007. Health Phys. 1982. PMID: 7174330
-
Calibration and measurement issues for therapeutic ultrasound.Ultrasonics. 2008 Aug;48(4):234-52. doi: 10.1016/j.ultras.2007.10.010. Epub 2008 Jan 8. Ultrasonics. 2008. PMID: 18234261 Review.
-
Metrology for ultrasonic applications.Prog Biophys Mol Biol. 2007 Jan-Apr;93(1-3):138-52. doi: 10.1016/j.pbiomolbio.2006.07.023. Epub 2006 Aug 10. Prog Biophys Mol Biol. 2007. PMID: 17081597 Review.
Cited by
-
Blisters on the anterior shin in 3 research subjects after a 1-MHz, 1.5-W/cm , continuous ultrasound treatment: a case series.J Athl Train. 2007 Jul-Sep;42(3):425-30. J Athl Train. 2007. PMID: 18060000 Free PMC article.
-
Intramuscular temperature differences between the mid-point and peripheral effective radiating area with ultrasound.J Sports Sci Med. 2008 Jun 1;7(2):286-91. eCollection 2008. J Sports Sci Med. 2008. PMID: 24149462 Free PMC article.
-
Effects of deep heating provided by therapeutic ultrasound on demyelinating nerves.J Phys Ther Sci. 2016 Apr;28(4):1278-83. doi: 10.1589/jpts.28.1278. Epub 2016 Apr 28. J Phys Ther Sci. 2016. PMID: 27190467 Free PMC article.
References
-
- Performance standards for sonic, infrasonic, and ultrasonic radiation emitting products. Food and Drug Administration. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFRCFRSearch.cfm?CFR.... Accessed September 22, 2006.
-
- Fyfe MC, Parnell SM. The importance of measurement of effective transducers radiating area in the testing and calibration of “therapeutic” ultrasonic instruments. Health Phys. 1982;43:377–381. - PubMed
-
- AS T40-1969 for Ultrasonic Therapeutic Equipment. Parkville, Victoria, Australia: Standards Association of Australia; 3052.
-
- International Electrotechnical Commission. Testing and Calibration of Ultrasonic Therapeutic Equipment. Geneva, Switzerland: Bureau Central de la Commission Electrotechnique Internationale; 1963: Publication 150.
-
- Johns LD, Straub SJ, Howard SM. Analysis of ERA, power, intensity and field characteristics of ultrasound transducers. Arch Phys Med Rehabil. 2007;88:124–129. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources