Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study
- PMID: 17598166
- PMCID: PMC6871022
- DOI: 10.1002/hbm.20428
Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study
Abstract
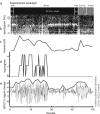
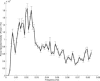
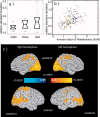
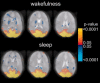
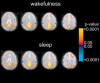
Recent blood oxygenation level dependent functional MRI (BOLD fMRI) studies of the human brain have shown that in the absence of external stimuli, activity persists in the form of distinct patterns of temporally correlated signal fluctuations. In this work, we investigated the spontaneous BOLD signal fluctuations during states of reduced consciousness such as drowsiness and sleep. For this purpose, we performed BOLD fMRI on normal subjects during varying levels of consciousness, from resting wakefulness to light (non-slow wave) sleep. Depth of sleep was determined based on concurrently acquired EEG data. During light sleep, significant increases in the fluctuation level of the BOLD signal were observed in several cortical areas, among which visual cortex was the most significant. Correlations among brain regions involved with the default-mode network persisted during light sleep. These results suggest that activity in areas such as the default-mode network and primary sensory cortex, as measured from BOLD fMRI fluctuations, does not require a level of consciousness typical of wakefulness.
Copyright 2007 Wiley-Liss, Inc.
Figures
References
-
- Allen PJ, Josephs O, Turner R ( 2000): A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 12: 230–239. - PubMed
-
- Andersson JL, Onoe H, Hetta J, Lidstrom K, Valind S, Lilja A, Sundin A, Fasth KJ, Westerberg G, Broman JE, Watanabe Y, Langstrom B. ( 1998): Brain networks affected by synchronized sleep visualized by positron emission tomography. J Cereb Blood Flow Metab 18: 701–715. - PubMed
-
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, Meyerand ME ( 2000): Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging 18: 921–930. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A ( 1996): Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 273: 1868–1871. - PubMed
-
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS ( 1992): Time course EPI of human brain function during task activation. Magn Reson Med 25: 390–397. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

