Inhibition of HIV-1 entry by antibodies: potential viral and cellular targets
- PMID: 17598813
- PMCID: PMC2367133
- DOI: 10.1111/j.1365-2796.2007.01820.x
Inhibition of HIV-1 entry by antibodies: potential viral and cellular targets
Abstract
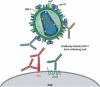
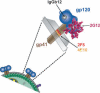
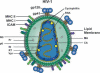
Vaccine-induced antibodies that interfere with viral entry are the protective correlate of most existing prophylactic vaccines. However, for highly variable viruses such as HIV-1, the ability to elicit broadly neutralizing antibody responses through vaccination has proven to be extremely difficult. The major targets for HIV-1 neutralizing antibodies are the viral envelope glycoprotein trimers on the surface of the virus that mediate receptor binding and entry. HIV-1 has evolved many mechanisms on the surface of envelope glycoproteins to evade antibody-mediated neutralization, including the masking of conserved regions by glycan, quaternary protein interactions and the presence of immunodominant variable elements. The primary challenge in the development of an HIV-1 vaccine that elicits broadly neutralizing antibodies therefore lies in the design of suitable envelope glycoprotein immunogens that circumvent these barriers. Here, we describe neutralizing determinants on the viral envelope glycoproteins that are defined by their function in receptor binding or by rare neutralizing antibodies isolated from HIV-infected individuals. We also describe the nonvariable cellular receptors involved in the HIV-1 entry process, or other cellular proteins, and ongoing studies to determine if antibodies against these proteins have efficacy as therapeutic reagents or, in some cases, as vaccine targets to interfere with HIV-1 entry.
Figures
Similar articles
-
Neutralizing Antibody Responses Induced by HIV-1 Envelope Glycoprotein SOSIP Trimers Derived from Elite Neutralizers.J Virol. 2020 Nov 23;94(24):e01214-20. doi: 10.1128/JVI.01214-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999024 Free PMC article.
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
Membrane Env Liposomes Facilitate Immunization with Multivalent Full-Length HIV Spikes.J Virol. 2021 Jun 10;95(13):e0000521. doi: 10.1128/JVI.00005-21. Epub 2021 Jun 10. J Virol. 2021. PMID: 33883221 Free PMC article.
-
GP120: target for neutralizing HIV-1 antibodies.Annu Rev Immunol. 2006;24:739-69. doi: 10.1146/annurev.immunol.24.021605.090557. Annu Rev Immunol. 2006. PMID: 16551265 Review.
-
The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens.Science. 1998 Jun 19;280(5371):1884-8. doi: 10.1126/science.280.5371.1884. Science. 1998. PMID: 9632381 Review.
Cited by
-
Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region.J Virol. 2009 Nov;83(21):11265-74. doi: 10.1128/JVI.01359-09. Epub 2009 Aug 19. J Virol. 2009. PMID: 19692477 Free PMC article.
-
Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C.J Virol. 2008 Dec;82(23):11651-68. doi: 10.1128/JVI.01762-08. Epub 2008 Sep 24. J Virol. 2008. PMID: 18815292 Free PMC article.
-
Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies.J Virol. 2008 May;82(9):4638-46. doi: 10.1128/JVI.00143-08. Epub 2008 Feb 27. J Virol. 2008. PMID: 18305047 Free PMC article.
-
The hepatitis C virus glycan shield and evasion of the humoral immune response.Viruses. 2011 Oct;3(10):1909-32. doi: 10.3390/v3101909. Epub 2011 Oct 14. Viruses. 2011. PMID: 22069522 Free PMC article. Review.
-
Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC.Virology. 2010 Dec 5;408(1):1-13. doi: 10.1016/j.virol.2010.08.028. Epub 2010 Sep 21. Virology. 2010. PMID: 20863545 Free PMC article.
References
-
- Berman PW, Gregory TJ, Riddle L, et al. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–5. - PubMed
-
- Ferrantelli F, Rasmussen RA, Hofmann-Lehmann R, Xu W, McClure HM, Ruprecht RM. Do not underestimate the power of antibodies – lessons from adoptive transfer of antibodies against HIV. Vaccine. 2002;20(Suppl 4):A61–5. - PubMed
-
- Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

