Sequential (gemcitabine/vinorelbine) and concurrent (gemcitabine) radiochemotherapy with FDG-PET-based target volume definition in locally advanced non-small cell lung cancer: first results of a phase I/II study
- PMID: 17598906
- PMCID: PMC1931600
- DOI: 10.1186/1471-2407-7-112
Sequential (gemcitabine/vinorelbine) and concurrent (gemcitabine) radiochemotherapy with FDG-PET-based target volume definition in locally advanced non-small cell lung cancer: first results of a phase I/II study
Abstract
Background: The aim of the study was to determine the maximal tolerated dose (MTD) of gemcitabine every two weeks concurrent to radiotherapy, administered during an aggressive program of sequential and simultaneous radiochemotherapy for locally advanced, unresectable non-small cell lung cancer (NSCLC) and to evaluate the efficacy of this regime in a phase II study.
Methods: 33 patients with histologically confirmed NSCLC were enrolled in a combined radiochemotherapy protocol. 29 patients were assessable for evaluation of toxicity and tumor response. Treatment included two cycles of induction chemotherapy with gemcitabine (1200 mg/m2) and vinorelbine (30 mg/m2) at day 1, 8 and 22, 29 followed by concurrent radiotherapy (2.0 Gy/d; total dose 66.0 Gy) and chemotherapy with gemcitabine every two weeks at day 43, 57 and 71. Radiotherapy planning included [18F] fluorodeoxyglucose positron emission tomography (FDG PET) based target volume definition. 10 patients were included in the phase I study with an initial gemcitabine dose of 300 mg/m2. The dose of gemcitabine was increased in steps of 100 mg/m2 until the MTD was realized.
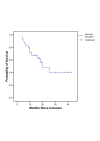
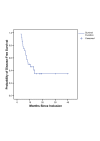
Results: MTD was defined for the patient group receiving gemcitabine 500 mg/m2 due to grade 2 (next to grade 3) esophagitis in all patients resulting in a mean body weight loss of 5 kg (SD = 1.4 kg), representing 8% of the initial weight. These patients showed persisting dysphagia 3 to 4 weeks after completing radiotherapy. In accordance with expected complications as esophagitis, dysphagia and odynophagia, we defined the MTD at this dose level, although no dose limiting toxicity (DLT) grade 3 was reached. In the phase I/II median follow-up was 15.7 months (4.1 to 42.6 months). The overall response rate after completion of therapy was 64%. The median overall survival was 19.9 (95% CI: [10.1; 29.7]) months for all eligible patients. The median disease-free survival for all patients was 8.7 (95% CI: [2.7; 14.6]) months.
Conclusion: After induction chemotherapy, the maximum tolerated dose and frequency of gemcitabine was defined at 500 mg/m2 every two weeks in three cycles during a maximum of 7 weeks of thoracic radiotherapy for the phase II study. This regimen represents an effective and tolerable therapy in the treatment of NSCLC.
Figures
Similar articles
-
Gemcitabine concurrent with thoracic radiotherapy after induction chemotherapy with gemcitabine/vinorelbine in locally advanced non-small cell lung cancer: a phase I study.Strahlenther Onkol. 2006 May;182(5):263-9. doi: 10.1007/s00066-006-1485-0. Strahlenther Onkol. 2006. PMID: 16673059 Clinical Trial.
-
A phase I/II trial of induction chemotherapy with carboplatin and gemcitabine followed by concurrent vinorelbine and paclitaxel with chest radiation in patients with stage III non-small cell lung cancer.Lung Cancer. 2004 Aug;45(2):243-53. doi: 10.1016/j.lungcan.2004.02.008. Lung Cancer. 2004. PMID: 15246197 Clinical Trial.
-
Phase I/IIa study of cisplatin and gemcitabine as induction chemotherapy followed by concurrent chemoradiotherapy with gemcitabine and paclitaxel for locally advanced non-small-cell lung cancer.J Clin Oncol. 2005 Sep 20;23(27):6664-73. doi: 10.1200/JCO.2005.02.519. J Clin Oncol. 2005. PMID: 16170174 Clinical Trial.
-
Options in advanced non-small cell lung cancer: a review and report on a phase II study of vinorelbine plus gemcitabine.Oncologist. 2001;6 Suppl 1:16-9. doi: 10.1634/theoncologist.6-suppl_1-16. Oncologist. 2001. PMID: 11182000 Review.
-
Gemcitabine and vinorelbine combinations in the treatment of non-small cell lung cancer.Semin Oncol. 1999 Oct;26(5 Suppl 16):67-70; discussion 71-2. Semin Oncol. 1999. PMID: 10585011 Review.
References
-
- Albain KS, Crowley JJ, Turrisi AT, III, Gandara DR, Farrar WB, Clark JI, Beasley KR, Livingston RB. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454–3460. doi: 10.1200/JCO.2002.03.055. - DOI - PubMed
-
- Armstrong JG. Target volume definition for three-dimensional conformal radiation therapy of lung cancer. Br J Radiol. 1998;71:587–594. - PubMed
-
- Choy H, Akerley W, Safran H, Graziano S, Chung C, Williams T, Cole B, Kennedy T. Multi-institutional phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 1998;16:3316–3322. - PubMed
-
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404 ff. doi: 10.1093/biomet/26.4.404. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical