PtdIns(4,5)P-restricted plasma membrane localization of FAN is involved in TNF-induced actin reorganization
- PMID: 17599063
- PMCID: PMC1933409
- DOI: 10.1038/sj.emboj.7601778
PtdIns(4,5)P-restricted plasma membrane localization of FAN is involved in TNF-induced actin reorganization
Abstract
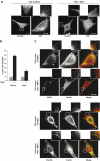
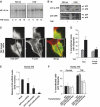
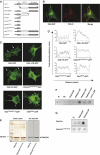
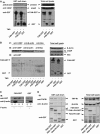
The WD-repeat protein factor associated with nSMase activity (FAN) is a member of the family of TNF receptor adaptor proteins that are coupled to specific signaling cascades. However, the precise functional involvement of FAN in specific cellular TNF responses remain unclear. Here, we report the involvement of FAN in TNF-induced actin reorganization and filopodia formation mediated by activation of Cdc42. The pleckstrin-homology (PH) domain of FAN specifically binds to phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P), which targets FAN to the plasma membrane. Site-specific mutagenesis revealed that the ability of FAN to mediate filopodia formation was blunted either by the destruction of the PtdIns(4,5)P binding motif, or by the disruption of intramolecular interactions between the PH domain and the adjacent beige and Chediak-Higashi (BEACH) domain. Furthermore, FAN was shown to interact with the actin cytoskeleton in TNF-stimulated cells via direct filamentous actin (F-actin) binding. The results of this study suggest that PH-mediated plasma membrane targeting of FAN is critically involved in TNF-induced Cdc42 activation and cytoskeleton reorganization.
Figures



References
-
- Abramoff M, Magelhaes P, Ram S (2004) Image processing with ImageJ. Biophotonics International 11: 36–42
-
- Adam D, Wiegmann K, Adam-Klages S, Ruff A, Krönke M (1996) A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. J Biol Chem 271: 14617–14622 - PubMed
-
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Krönke M (1996) FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell 86: 937–947 - PubMed
-
- Adam-Klages S, Schwandner R, Adam D, Kreder D, Bernardo K, Krönke M (1998) Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J Leukoc Biol 63: 678–682 - PubMed
-
- Banno T, Gazel A, Blumenberg M (2004) Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem 279: 32633–32642 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

