Functional dynamics of H3K9 methylation during meiotic prophase progression
- PMID: 17599069
- PMCID: PMC1933398
- DOI: 10.1038/sj.emboj.7601767
Functional dynamics of H3K9 methylation during meiotic prophase progression
Abstract
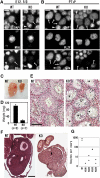
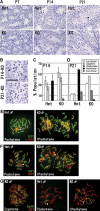
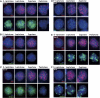
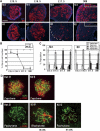
Histone H3 lysine 9 (H3K9) methylation is a crucial epigenetic mark of heterochromatin formation and transcriptional silencing. G9a is a major mammalian H3K9 methyltransferase at euchromatin and is essential for mouse embryogenesis. Here we describe the roles of G9a in germ cell development. Mutant mice in which G9a is specifically inactivated in the germ-lineage displayed sterility due to a drastic loss of mature gametes. G9a-deficient germ cells exhibited perturbation of synchronous synapsis in meiotic prophase. Importantly, mono- and di-methylation of H3K9 (H3K9me1 and 2) in G9a-deficient germ cells were significantly reduced and G9a-regulated genes were overexpressed during meiosis, suggesting that G9a-mediated epigenetic gene silencing is crucial for proper meiotic prophase progression. Finally, we show that H3K9me1 and 2 are dynamically and sex-differentially regulated during the meiotic prophase. This genetic and biochemical evidence strongly suggests that a specific set of H3K9 methyltransferase(s) and demethylase(s) coordinately regulate gametogenesis.
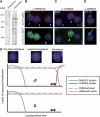
Figures



References
-
- Bourc'his D, Bestor TH (2004) Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431: 96–99 - PubMed
-
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE (2004) Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36: 647–652 - PubMed
-
- Cao R, Zhang Y (2004) The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 14: 155–164 - PubMed
-
- Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ (2007) MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12: 503–514 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

