The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation
- PMID: 17599095
- PMCID: PMC3345951
- DOI: 10.1038/sj.cdd.4402194
The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation
Abstract
Pyroptosis is a caspase-1-dependent inflammatory form of cell death. The adapter protein ASC binds directly to caspase-1 and is critical for caspase-1 activation in response to a broad range of stimuli. To elucidate the mechanism of activation of caspase-1 by ASC and its exact role in macrophage pyroptosis, we performed time-lapse confocal bioimaging analysis on human THP-1 macrophages stably expressing an ASC-GFP fusion protein. We show that stimulation of these cells with several proinflammatory stimuli trigger the formation of a large supramolecular assembly of ASC, termed here pyroptosome. Only one distinct pyroptosome in each stimulated cell is formed, which rapidly recruits and activates caspase-1 resulting in pyroptosis and the release of the intracellular proinflammatory cytokines. The pyroptosome is largely composed of oligomerized ASC dimers. Dimerization of ASC is driven by subphysiological concentrations of potassium as in vitro incubation of purified recombinant ASC in the presence of subphysiological concentrations of potassium induces the assembly of a functional pyroptosome. Furthermore, stimulation of potassium efflux in THP-1 cells with potassium-depleting agents induces formation of the pyroptosome, while increasing potassium concentrations in the culture medium or pharmacological inhibition of this efflux inhibits its assembly. Our results establish that macrophage pyroptosis is mediated by a unique pyroptosome, distinct from the inflammasome.
Figures
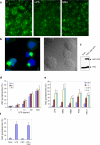
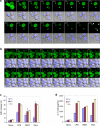

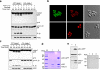

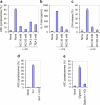


Comment in
-
Caspase-1 inflammasomes: choosing between death and taxis.Cell Death Differ. 2007 Sep;14(9):1559-60. doi: 10.1038/sj.cdd.4402203. Cell Death Differ. 2007. PMID: 17703235 No abstract available.
References
-
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. - PubMed
-
- Ting JP, Kastner DL, Hoffman HM. Caterpillers, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. - PubMed
-
- Delbridge LM, O'Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. - PubMed
-
- Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. - PubMed
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proI-Lbeta. Mol Cell. 2002;10:417–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

