Modulation of Aire regulates the expression of tissue-restricted antigens
- PMID: 17599412
- PMCID: PMC1994210
- DOI: 10.1016/j.molimm.2007.05.014
Modulation of Aire regulates the expression of tissue-restricted antigens
Abstract
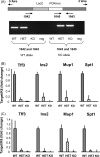
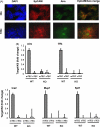
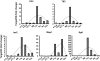
Intrathymic expression of tissue-restricted antigens (TRAs) has been viewed as the key element in the induction of central tolerance and recently, a central role for the autoimmune regulator (Aire) has been suggested in this process. The aim of this study was to establish whether down or up-regulation of Aire leads to alterations in TRA expression and whether this is limited to thymic epithelial cells. This study also characterized whether TRAs follow Aire expression during normal development, and whether thymic microenvironment plays a role in the expression of Aire and TRAs. We did several in vivo and in vitro experiments to manipulate Aire expression and measured expression of four TRAs (Trefoil factor-3, Insulin-2, Major urinary protein-1 and Salivary protein-1) by real-time RT-PCR. Aire had an allele dose-dependent effect on TRA expression in the thymuses of mice from two strains, C57BL/6J and Balb/c, but had no effect on TRA expression in the lymph nodes. In the thymus, Aire and TRAs were both localized in the medulla and were co-expressed during normal development and involution. In the primary stromal cells as well as thymic epithelial cell line, the adenoviral over-expression of Aire resulted in an increase in TRA expression. By manipulating in vitro organ-cultures we showed that thymic microenvironment plays a dominant role in Aire expression whereas TRAs follow the same pattern. The data underline a direct role for Aire in TRA expression and suggest that modulation of Aire has a potential to control central tolerance and autoimmunity.
Figures



References
-
- Akiyama T., Maeda S., Yamane S., Ogino K., Kasai M., Kajiura F., Matsumoto M., Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308:248–251. - PubMed
-
- Anderson G., Jenkinson W.E., Jones T., Parnell S.M., Kinsella F.A., White A.J., Pongrac’z J.E., Rossi S.W., Jenkinson E.J. Establishment and functioning of intrathymic microenvironments. Immunol. Rev. 2006;209:10–27. - PubMed
-
- Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., von Boehmer H., Bronson R., Dierich A., Benoist C., Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
-
- Bjorses P., Pelto-Huikko M., Kaukonen J., Aaltonen J., Peltonen L., Ulmanen I. Localization of the APECED protein in distinct nuclear structures. Hum. Mol. Genet. 1999;8:259–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

