Reperfusion accelerates acute neuronal death induced by simulated ischemia
- PMID: 17599834
- PMCID: PMC3648807
- DOI: 10.1016/j.expneurol.2007.05.017
Reperfusion accelerates acute neuronal death induced by simulated ischemia
Abstract
Observations in real time can provide insights into the timing of injury and the mechanisms of damage in neural ischemia-reperfusion. Continuous digital imaging of morphology and cell viability was applied in a novel model of simulated ischemia-reperfusion in cultured cortical neurons, consisting of exposure to severe hypoxia combined with glucose deprivation, mild acidosis, hypercapnia, and elevated potassium, followed by return of oxygenated, glucose-containing physiological saline. Substantial acute injury resulted following 1 h of simulated ischemia, with 36+/-8% neurons dying within 2 h of reperfusion. Inclusion of moderate glutamate elevation (30 microM) in the simulation of ischemia increased the acute neuronal death to 51+/-6% at 2 h of reperfusion. While some swelling and neuritic breakdown occurred during ischemia, particularly with inclusion of glutamate, neuronal death, as marked by loss of somatic membrane integrity, was entirely restricted to the reperfusion phase. Morphological and cytoskeletal changes suggested a predominance of necrotic death in the acute phase of reperfusion, with more complete delayed death accompanied by some apoptotic features occurring over subsequent days. Prolonged simulated ischemia, without reperfusion, did not induce significant acute neuronal death even when extended to 3 h. We conclude that while morphological changes suggesting initiation of neuronal injury appear during severe simulated ischemia, the irreversible injury signaled by membrane breakdown is accelerated by the events of reperfusion itself.
Figures

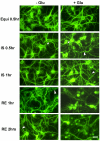
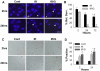

References
-
- Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;4:413–419. - PubMed
-
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. - PubMed
-
- Ambrosio G, Flaherty JT. Effects of the superoxide radical scavenger superoxide dismutase, and of the hydroxyl radical scavenger mannitol, on reperfusion injury in isolated rabbit hearts. Cardiovasc. Drugs Ther. 1992;6:623–632. - PubMed
-
- Aronowski J, Strong R, Grotta J. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 1997;17:1048–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

