Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex
- PMID: 17599963
- PMCID: PMC2075248
- DOI: 10.1113/jphysiol.2007.134577
Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex
Abstract
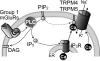
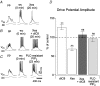
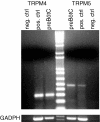
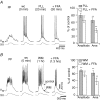
Neurons of the preBötzinger complex (preBötC) form local excitatory networks and synchronously discharge bursts of action potentials during the inspiratory phase of respiratory network activity. Synaptic input periodically evokes a Ca(2+)-activated non-specific cation current (I(CAN)) postsynaptically to generate 10-30 mV transient depolarizations, dubbed inspiratory drive potentials, which underlie inspiratory bursts. The molecular identity of I(CAN) and its regulation by intracellular signalling mechanisms during inspiratory drive potential generation remains unknown. Here we show that mRNAs coding for two members of the transient receptor potential (TRP) family of ion channels, namely TRPM4 and TRPM5, are expressed within the preBötC region of neonatal mice. Hypothesizing that the phosphoinositides maintaining TRPM4 and TRPM5 channel sensitivity to Ca(2+) may similarly influence I(CAN) and thus regulate inspiratory drive potentials, we manipulated intracellular phosphatidylinositol 4,5-bisphosphate (PIP(2)) and measured its effect on preBötC neurons in the context of ongoing respiratory-related rhythms in slice preparations. Consistent with the involvement of TRPM4 and TRPM5, excess PIP(2) augmented the inspiratory drive potential and diminution of PIP(2) reduced it; sensitivity to flufenamic acid (FFA) suggested that these effects of PIP(2) were I(CAN) mediated. Inositol 1,4,5-trisphosphate (IP(3)), the product of PIP(2) hydrolysis, ordinarily causes IP(3) receptor-mediated I(CAN) activation. Simultaneously increasing PIP(2) while blocking IP(3) receptors intracellularly counteracted the reduction in the inspiratory drive potential that normally resulted from IP(3) receptor blockade. We propose that PIP(2) protects I(CAN) from rundown by interacting directly with underlying ion channels and preventing desensitization, which may enhance the robustness of respiratory rhythm.
Figures

Similar articles
-
Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice.J Physiol. 2007 Jul 1;582(Pt 1):113-25. doi: 10.1113/jphysiol.2007.133660. Epub 2007 Apr 19. J Physiol. 2007. PMID: 17446214 Free PMC article.
-
Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm.Neuroscience. 2005;130(4):1069-81. doi: 10.1016/j.neuroscience.2004.10.028. Neuroscience. 2005. PMID: 15653001 Free PMC article.
-
Role of persistent sodium current in mouse preBötzinger Complex neurons and respiratory rhythm generation.J Physiol. 2007 Apr 15;580(Pt. 2):485-96. doi: 10.1113/jphysiol.2006.124602. Epub 2007 Feb 1. J Physiol. 2007. PMID: 17272351 Free PMC article.
-
Somatic Ca2+ transients do not contribute to inspiratory drive in preBötzinger Complex neurons.J Physiol. 2008 Sep 15;586(18):4531-40. doi: 10.1113/jphysiol.2008.154765. Epub 2008 Jul 17. J Physiol. 2008. PMID: 18635649 Free PMC article.
-
Regulation of transient receptor potential (TRP) channels by phosphoinositides.Pflugers Arch. 2007 Oct;455(1):157-68. doi: 10.1007/s00424-007-0275-6. Epub 2007 May 4. Pflugers Arch. 2007. PMID: 17479281 Review.
Cited by
-
Cooperation of intrinsic bursting and calcium oscillations underlying activity patterns of model pre-Bötzinger complex neurons.J Comput Neurosci. 2013 Apr;34(2):345-66. doi: 10.1007/s10827-012-0425-5. Epub 2012 Sep 28. J Comput Neurosci. 2013. PMID: 23053862
-
Putting the theory into 'burstlet theory' with a biophysical model of burstlets and bursts in the respiratory preBötzinger complex.Elife. 2022 Apr 5;11:e75713. doi: 10.7554/eLife.75713. Elife. 2022. PMID: 35380537 Free PMC article.
-
Rhythmic bursting in the pre-Bötzinger complex: mechanisms and models.Prog Brain Res. 2014;209:1-23. doi: 10.1016/B978-0-444-63274-6.00001-1. Prog Brain Res. 2014. PMID: 24746040 Free PMC article.
-
TRPM4-dependent post-synaptic depolarization is essential for the induction of NMDA receptor-dependent LTP in CA1 hippocampal neurons.Pflugers Arch. 2016 Apr;468(4):593-607. doi: 10.1007/s00424-015-1764-7. Epub 2015 Dec 3. Pflugers Arch. 2016. PMID: 26631168 Free PMC article.
-
TRPC channels are not required for graded persistent activity in entorhinal cortex neurons.Hippocampus. 2019 Nov;29(11):1038-1048. doi: 10.1002/hipo.23094. Epub 2019 Apr 19. Hippocampus. 2019. PMID: 31002217 Free PMC article.
References
-
- Balla T. Phosphatidylinositol 4-kinases. Biochim Biophys Acta. 1998;1436:69–85. - PubMed
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
-
- Buzsáki G. Rhythms of the Brain. Oxford, New York: Oxford University Press; 2006.
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
-
- Enklaar T, Esswein M, Oswald M, Hilbert K, Winterpacht A, Higgins M, Zabel B, Prawitt D. Mtr1, a novel biallelically expressed gene in the center of the mouse distal chromosome 7 imprinting cluster, is a member of the Trp gene family. Genomics. 2000;67:179–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

