Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells
- PMID: 17600088
- PMCID: PMC1913903
- DOI: 10.1073/pnas.0703040104
Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells
Abstract
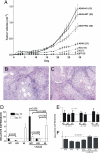
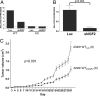
Integrin alpha11 (ITGA11/alpha11) is localized to stromal fibroblasts and commonly overexpressed in non-small-cell lung carcinoma (NSCLC). We hypothesized that stromal alpha11 could be important for the tumorigenicity of NSCLC cells. SV40 immortalized mouse embryonic fibroblasts established from wild-type (WT) and Itga11-deficient [knockout (KO)] mice were tested for their tumorigenicity in immune-deficient mice when implanted alone or coimplanted with the A549 human lung adenocarcinoma cells. A549 coimplanted with the fibroblasts showed a markedly enhanced tumor growth rate compared with A549, WT, or KO, which alone formed only small tumors. Importantly, the growth was significantly greater for A549+WT compared with A549+KO tumors. Reexpression of human alpha11 cDNA in KO cells rescued a tumor growth rate to that comparable with the A549+WT tumors. These findings were validated in two other NSCLC cell lines, NCI-H460 and NCI-H520. Gene expression profiling indicated that IGF2 mRNA expression level was >200 times lower in A549+KO compared with A549+WT tumors. Stable short-hairpin RNA (shRNA) down-regulation of IGF2 in WT (WT(shIGF2)) fibroblasts resulted in a decreased growth rate of A549+WT(shIGF2), compared with A549+WT tumors. The results indicate that alpha11 is an important stromal factor in NSCLC and propose a paradigm for carcinoma-stromal interaction indirectly through interaction between the matrix collagen and stromal fibroblasts to stimulate cancer cell growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer.Oncogene. 2016 Apr 14;35(15):1899-908. doi: 10.1038/onc.2015.254. Epub 2015 Jul 6. Oncogene. 2016. PMID: 26148229 Free PMC article.
-
[shRNA-mediated insulin-like growth factor I receptor gene silencing inhibits cell proliferation, induces cell apoptosis, and suppresses tumor growth in non-small cell lung cancer: in vitro and in vivo experiments].Zhonghua Yi Xue Za Zhi. 2007 Jun 5;87(21):1506-9. Zhonghua Yi Xue Za Zhi. 2007. PMID: 17785094 Chinese.
-
Down-regulation of hepatoma-derived growth factor inhibits anchorage-independent growth and invasion of non-small cell lung cancer cells.Cancer Res. 2006 Jan 1;66(1):18-23. doi: 10.1158/0008-5472.CAN-04-3905. Cancer Res. 2006. PMID: 16397209
-
Cancer-stromal interaction through growth factor/cytokine networks implicated in growth of stomach cancer.Princess Takamatsu Symp. 1994;24:187-94. Princess Takamatsu Symp. 1994. PMID: 8983075 Review.
-
Mouse models for human lung cancer.Genes Dev. 2005 Mar 15;19(6):643-64. doi: 10.1101/gad.1284505. Genes Dev. 2005. PMID: 15769940 Review.
Cited by
-
Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer.Oncogene. 2016 Apr 14;35(15):1899-908. doi: 10.1038/onc.2015.254. Epub 2015 Jul 6. Oncogene. 2016. PMID: 26148229 Free PMC article.
-
COL1A1 expression induced by overexpression of both a 15‑amino acid peptide from the fibrinogen domain of tenascin‑X and integrin α11 in LX‑2 cells.Mol Med Rep. 2022 Nov;26(5):330. doi: 10.3892/mmr.2022.12846. Epub 2022 Sep 7. Mol Med Rep. 2022. PMID: 36069233 Free PMC article.
-
Cancer-associated fibroblast migration in non-small cell lung cancers is modulated by increased integrin α11 expression.Mol Oncol. 2021 May;15(5):1507-1527. doi: 10.1002/1878-0261.12937. Epub 2021 Mar 25. Mol Oncol. 2021. PMID: 33682233 Free PMC article.
-
Dwarfism in mice lacking collagen-binding integrins α2β1 and α11β1 is caused by severely diminished IGF-1 levels.J Biol Chem. 2012 Feb 24;287(9):6431-40. doi: 10.1074/jbc.M111.283119. Epub 2011 Dec 30. J Biol Chem. 2012. PMID: 22210772 Free PMC article.
-
The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation.J Biol Chem. 2010 Apr 2;285(14):10434-45. doi: 10.1074/jbc.M109.078766. Epub 2010 Feb 2. J Biol Chem. 2010. PMID: 20129924 Free PMC article.
References
-
- Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Cancer Res. 1991;51:3753–3761. - PubMed
-
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. Science. 2004;303:848–851. - PubMed
-
- Muerkoster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Kloppel G, Kalthoff H, Folsch UR, Schafer H. Cancer Res. 2004;64:1331–1337. - PubMed
-
- Micke P, Ostman A. Lung Cancer. 2004;45(Suppl 2):S163–S175. - PubMed
-
- Mueller MM, Fusenig NE. Nat Rev Cancer. 2004;4:839–849. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

