Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease
- PMID: 17600089
- PMCID: PMC1913907
- DOI: 10.1073/pnas.0700574104
Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease
Abstract
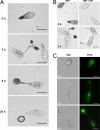
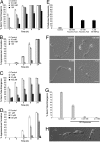
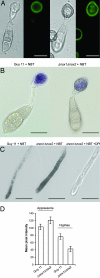
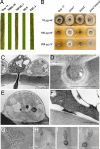
One of the first responses of plants to microbial attack is the production of extracellular superoxide surrounding infection sites. Here, we report that Magnaporthe grisea, the causal agent of rice blast disease, undergoes an oxidative burst of its own during plant infection, which is associated with its development of specialized infection structures called appressoria. Scavenging of these oxygen radicals significantly delayed the development of appressoria and altered their morphology. We targeted two superoxide-generating NADPH oxidase-encoding genes, Nox1 and Nox2, and demonstrated genetically, that each is independently required for pathogenicity of M. grisea. Deltanox1 and Deltanox2 mutants are incapable of causing plant disease because of an inability to bring about appressorium-mediated cuticle penetration. The initiation of rice blast disease therefore requires production of superoxide by the invading pathogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
NADPH oxidases regulate septin-mediated cytoskeletal remodeling during plant infection by the rice blast fungus.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3179-84. doi: 10.1073/pnas.1217470110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382235 Free PMC article.
-
A fungal metallothionein is required for pathogenicity of Magnaporthe grisea.Plant Cell. 2004 Jun;16(6):1575-88. doi: 10.1105/tpc.021279. Epub 2004 May 21. Plant Cell. 2004. PMID: 15155887 Free PMC article.
-
Magnaporthe grisea cutinase2 mediates appressorium differentiation and host penetration and is required for full virulence.Plant Cell. 2007 Aug;19(8):2674-89. doi: 10.1105/tpc.107.051219. Epub 2007 Aug 17. Plant Cell. 2007. PMID: 17704215 Free PMC article.
-
The molecular biology of appressorium turgor generation by the rice blast fungus Magnaporthe grisea.Biochem Soc Trans. 2005 Apr;33(Pt 2):384-8. doi: 10.1042/BST0330384. Biochem Soc Trans. 2005. PMID: 15787612 Review.
-
Every Coin Has Two Sides: Reactive Oxygen Species during Rice⁻Magnaporthe oryzae Interaction.Int J Mol Sci. 2019 Mar 8;20(5):1191. doi: 10.3390/ijms20051191. Int J Mol Sci. 2019. PMID: 30857220 Free PMC article. Review.
Cited by
-
The NADPH oxidase complexes in Botrytis cinerea: evidence for a close association with the ER and the tetraspanin Pls1.PLoS One. 2013;8(2):e55879. doi: 10.1371/journal.pone.0055879. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418468 Free PMC article.
-
Genome Analysis of the Broad Host Range Necrotroph Nalanthamala psidii Highlights Genes Associated With Virulence.Front Plant Sci. 2022 Feb 25;13:811152. doi: 10.3389/fpls.2022.811152. eCollection 2022. Front Plant Sci. 2022. PMID: 35283890 Free PMC article.
-
Identification and characterization of Sclerotinia sclerotiorum NADPH oxidases.Appl Environ Microbiol. 2011 Nov;77(21):7721-9. doi: 10.1128/AEM.05472-11. Epub 2011 Sep 2. Appl Environ Microbiol. 2011. PMID: 21890677 Free PMC article.
-
MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae.Autophagy. 2018;14(9):1543-1561. doi: 10.1080/15548627.2018.1458171. Epub 2018 Aug 31. Autophagy. 2018. PMID: 29929416 Free PMC article.
-
Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex.Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2861-6. doi: 10.1073/pnas.1017309108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282602 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

