Negative regulation of MDA5- but not RIG-I-mediated innate antiviral signaling by the dihydroxyacetone kinase
- PMID: 17600090
- PMCID: PMC1913852
- DOI: 10.1073/pnas.0700544104
Negative regulation of MDA5- but not RIG-I-mediated innate antiviral signaling by the dihydroxyacetone kinase
Abstract
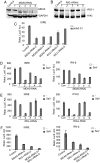
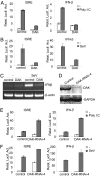
Viral infection leads to activation of the transcription factors interferon regulatory factor-3 and NF-kappaB, which collaborate to induce type I IFNs. The RNA helicase proteins RIG-I and MDA5 were recently identified as two cytoplasmic viral RNA sensors that recognize different species of viral RNAs produced during viral replication. In this study, we identified DAK, a functionally unknown dihydroacetone kinase, as a specific MDA5-interacting protein. DAK was associated with MDA5, but not RIG-I, under physiological conditions. Overexpression of DAK inhibited MDA5- but not RIG-I- or TLR3-mediated IFN-beta induction. Overexpression of DAK also inhibited cytoplasmic dsRNA and SeV-induced activation of the IFN-beta promoter, whereas knockdown of endogenous DAK by RNAi activated the IFN-beta promoter, and increased cytoplasmic dsRNA- or SeV-triggered activation of the IFN-beta promoter. In addition, overexpression of DAK inhibited MDA5- but not RIG-I-mediated antiviral activity, whereas DAK RNAi increased cytoplasmic dsRNA-triggered antiviral activity. These findings suggest that DAK is a physiological suppressor of MDA5 and specifically inhibits MDA5- but not RIG-I-mediated innate antiviral signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The ubiquitin-specific protease 17 is involved in virus-triggered type I IFN signaling.Cell Res. 2010 Jul;20(7):802-11. doi: 10.1038/cr.2010.41. Epub 2010 Apr 6. Cell Res. 2010. PMID: 20368735
-
RAVER1 is a coactivator of MDA5-mediated cellular antiviral response.J Mol Cell Biol. 2013 Apr;5(2):111-9. doi: 10.1093/jmcb/mjt006. Epub 2013 Feb 5. J Mol Cell Biol. 2013. PMID: 23390309
-
RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium.J Immunol. 2011 Feb 1;186(3):1618-26. doi: 10.4049/jimmunol.1002862. Epub 2010 Dec 27. J Immunol. 2011. PMID: 21187438
-
LGP2 synergy with MDA5 in RLR-mediated RNA recognition and antiviral signaling.Cytokine. 2015 Aug;74(2):198-206. doi: 10.1016/j.cyto.2015.02.010. Epub 2015 Mar 18. Cytokine. 2015. PMID: 25794939 Free PMC article. Review.
-
[Recognition of viral nucleic acids and regulation of type I IFN expression].Nihon Rinsho. 2006 Jul;64(7):1236-43. Nihon Rinsho. 2006. PMID: 16838638 Review. Japanese.
Cited by
-
Alternative Splicing of the Last TKFC Intron Yields Transcripts Differentially Expressed in Human Tissues That Code In Vitro for a Protein Devoid of Triokinase and FMN Cyclase Activity.Biomolecules. 2024 Oct 12;14(10):1288. doi: 10.3390/biom14101288. Biomolecules. 2024. PMID: 39456221 Free PMC article.
-
Sustained activation of interferon regulatory factor 3 during infection by paramyxoviruses requires MDA5.J Innate Immun. 2014;6(5):650-62. doi: 10.1159/000360764. Epub 2014 Apr 30. J Innate Immun. 2014. PMID: 24800889 Free PMC article.
-
Spotted Fever Group Rickettsia Trigger Species-Specific Alterations in Macrophage Proteome Signatures with Different Impacts in Host Innate Inflammatory Responses.Microbiol Spectr. 2021 Dec 22;9(3):e0081421. doi: 10.1128/spectrum.00814-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34935429 Free PMC article.
-
Induction of type I interferon by RNA viruses: cellular receptors and their substrates.Amino Acids. 2010 May;38(5):1283-99. doi: 10.1007/s00726-009-0374-0. Epub 2009 Nov 1. Amino Acids. 2010. PMID: 19882216 Free PMC article. Review.
-
Intracellular sensing of viral genomes and viral evasion.Exp Mol Med. 2019 Dec 11;51(12):1-13. doi: 10.1038/s12276-019-0299-y. Exp Mol Med. 2019. PMID: 31827068 Free PMC article. Review.
References
-
- Durbin JE, Fernandez-Sesma A, Lee CK, Rao TD, Frey AB, Moran TM, Vukmanovic S, Garcia-Sastre A, Levy DE. J Immunol. 2000;164:4220–4228. - PubMed
-
- Levy DE, Garcia-Sastre A. Cytokine Growth Factor Rev. 2001;12:143–156. - PubMed
-
- Levy DE, Marie IJ. Nat Immunol. 2004;5:699–701. - PubMed
-
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. Annu Rev Immunol. 2001;19:623–655. - PubMed
-
- Honda K, Takaoka A, Taniguchi T. Immunity. 2006;25:349–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

