Molecular changes in the vasculature of injured tissues
- PMID: 17600129
- PMCID: PMC1934529
- DOI: 10.2353/ajpath.2007.061251
Molecular changes in the vasculature of injured tissues
Abstract
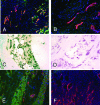
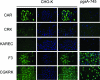
We have explored molecular specialization of the vasculature of regenerating wound tissue in the skin and tendons to identify a different repertoire of markers from that obtained by studying tumor vasculature. We screened a phage-displayed peptide library for peptides that home to wounds in mice and identified two peptides that selectively target phage to skin and tendon wounds: CARSKNKDC (CAR) and CRKDKC (CRK). CAR is homologous to heparin-binding sites in various proteins and binds to cell surface heparan sulfate and heparin. CRK is similar to a segment in thrombospondin type 1 repeat. Intravenously injected CAR and CRK phage, as well as fluorescein-labeled CAR and CRK peptides, selectively accumulated at wound sites, where they partially co-localized with blood vessels. The CAR peptide showed a preference for early stages of wound healing, whereas the CRK favored wounds at later stages of healing. The CAR peptide was internalized into the target cells and delivered the fluorescent label into the cell nuclei. These results identify new molecular markers in wound tissues and show that the expression of these markers in wound vasculature changes as healing progresses. The peptides recognizing these markers may be useful in delivering treatments into regenerating tissues.
Figures




References
-
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. - PubMed
-
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Wickström S, Keski-Oja J, Alitalo K. Matrix reloaded to circulation hits the tumor target. Cancer Cell. 2003;3:513–514. - PubMed
-
- St. Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

